This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New drug could unlock benefits of immunotherapy for more patients
A new drug could offer a powerful way to sensitize tumors to immunotherapy, a new trial suggests. The results have been published in the Journal of Clinical Investigation.
Ceralasertib showed promise for patients no longer responding to current cancer treatments in an early clinical trial. Given on its own, the drug stabilized the growth of tumors in more than half of patients who received it, with one patient seeing benefits for more than five years.
Prime tumors to be more responsive to immunotherapy
Researchers found that ceralasertib, a drug that targets cancer's ability to repair its DNA by blocking a key protein called ATR, also profoundly increased immune activity in some patient's tumors—changes which could leave them much more susceptible to immunotherapy treatments.
The study opens exciting new avenues for future trials which would use ceralasertib to prime tumors to be more responsive to immunotherapies, potentially unlocking their benefits for a wider group of patients.
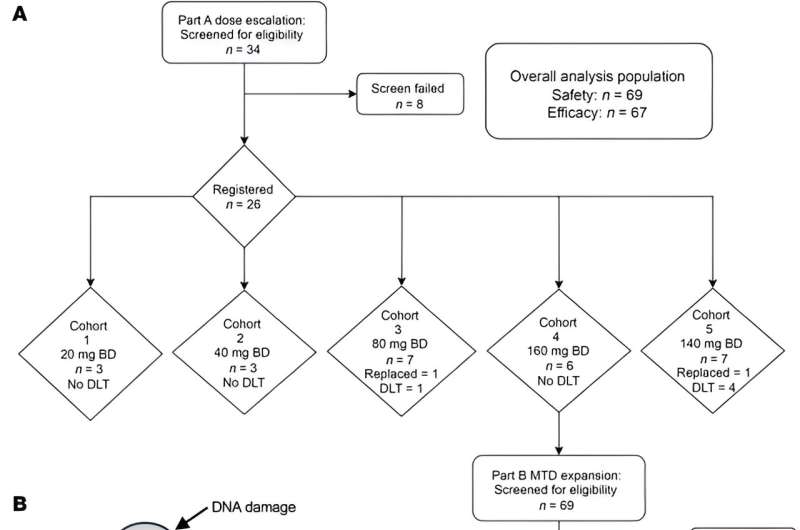
A team at The Institute of Cancer Research, London, and The Royal Marsden NHS Foundation Trust led the phase I PATRIOT trial. Researchers treated 67 patients with very advanced solid tumors with ceralasertib on its own. For these patients, other treatments had stopped working and their tumors were continuing to grow.
Tumor biopsies showed immune response
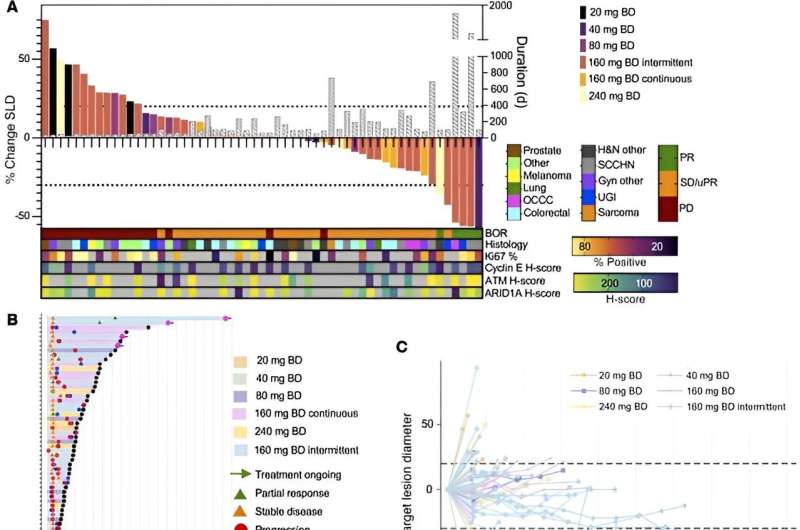
Ceralasertib, which can be taken as a pill, stopped tumors growing in more than half of patients—34 out of 66 patients whose treatment response could be measured. In five patients, the drug shrank their tumors. Of the 39 patients who benefited from the drug, 68% saw no progression of their disease for at least four months.
One patient with advanced ovarian cancer, whose tumor had faults in the key DNA repair gene ARID1A, responded remarkably well to ceralasertib, seeing their tumor continue to shrink over a period of more than five years. Researchers at The Institute of Cancer Research (ICR) previously identified the ARID1A gene as a marker of sensitivity to ATR inhibitors such as ceralasertib.
In the current study, the scientists compared tumor biopsies taken before and after treatment with ceralasertib to understand the underlying biology behind the drug's effects.
They were excited to observe that the drug on its own caused profound changes in patients' immune systems, both in their blood and within their tumors, in addition to its impact on DNA repair.
Giving ceralasertib led to increases in a type of immune cell that seeks out and kills cancer cells in the blood, alongside increased infiltration of immune cells into the tumors. These are signs that tumors are under attack and would be responsive to a range of immunotherapies—drugs which harness the body's own immune system to fight cancer.
Critical biological insights
Clinical trials have already shown that ceralasertib is effective when used in combination with the most common type of immunotherapy, known as PDL-1 inhibitors, but the current study is the first to prove that ceralasertib modulates the immune system in its own right.
The researchers have gained critical insight into the way in which the drug primes tumors to respond to immunotherapies. They hope this will lead to even better combinations of the drug with immunotherapy—a cutting-edge class of drugs which currently only works for a minority of patients.
Since the PATRIOT trial launched, other clinical trials led by the ICR and The Royal Marsden have begun—investigating the use of ceralasertib in combination with other drugs which prevent DNA from repairing itself, such as the PARP inhibitor olaparib.
Study leader Dr. Magnus Dillon, Clinician Scientist at The Institute of Cancer Research, London, and Clinical Consultant at The Royal Marsden NHS Foundation Trust, said, "This is the largest clinical trial of an ATR inhibitor, and it's encouraging to see that on its own ceralasertib can keep cancer from progressing and even shrink patients' tumors for an impressive time, giving some patients precious extra years of living well. It has also given us a clue as to the biological markers which may predict who could benefit from this drug in future.
"Excitingly, this trial provides us with the biological insights for how best to combine this drug with an immunotherapy and generate an even more powerful cancer treatment for people who have exhausted other options."
Dr. Anna Kinsella, Science Engagement Manager at Cancer Research U.K., said, "This is the largest study to date showing the potential of a class of drugs called ATR inhibitors in treating cancer when used alone. While there is still a long way to go before ceralasertib can be used in the clinic, it's always exciting to see new approaches showing potential in early-stage clinical trials.
"These promising results lay the foundations for future clinical trials and offer scientists and doctors new avenues of research. We look forward to seeing how this work drives further progress."
More information: Magnus T. Dillon et al, Durable responses to ATR inhibition with ceralasertib in tumors with genomic defects and high inflammation, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI175369