This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
How human cytomegalovirus hijacks the immune system
The human cytomegalovirus, HCMV for short, lies dormant unnoticed in the body of most people for their entire lives. In immunocompromised individuals, however, the virus can cause life-threatening infections. It infects dendritic cells, a specific type of cell in the immune system.
Although the majority of them are infected, only a few of them immediately execute the virus's genetic program. Researchers at TWINCORE, Centre for Experimental and Clinical Infection Research, have now been able to show which signaling pathways of the innate immune system the virus is targeting in order to have itself produced by the host cells.
They have published their findings in the journal Nature Communications.
Up to 90% of the world's population carries HCMV. In people with a normal immune system, the infection is usually subclinical, i.e. it does not cause any pronounced symptoms. However, in immunocompromised patients, such as organ transplant recipients, the infection can become life-threatening. In addition, infection with HCMV during pregnancy is the most common cause of malformations in newborns, such as congenital hearing impairment.
In the human body, HCMV can infect the myeloid cells of the immune system. This group also includes the dendritic cells derived from monocytes, whose main task is to process foreign proteins so that they can be presented to the T cells. Antigen-specific T cells can then eliminate infected cells.
The researchers from the Institute for Experimental Infection Research, led by Prof Ulrich Kalinke, were able to divide the monocyte-derived dendritic cells into three groups, one of which is more susceptible to infection than the others.
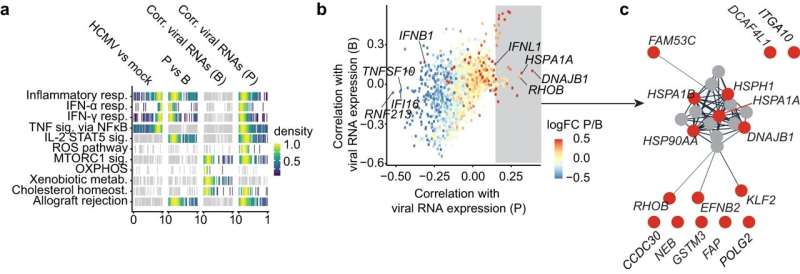
"Using single-cell RNA sequencing, we found that in these cells, the signaling pathway that normally recognizes viruses is virtually hijacked by HCMV in order to establish the productive infection," says Dr. Bibiana Costa, a postdoctoral researcher at TWINCORE and first author of the study. "This is the so-called STING signaling pathway." STING stands for "stimulator of interferon genes".
Interferons are messenger substances of the immune system that act directly against viruses and can also activate defense cells. In addition, interferons trigger further defense processes in a chain reaction. In the subgroup of dendritic cells, which are particularly susceptible to infection, the virus' own proteins block this protective function and instead reprogram them so that new virus particles are produced undisturbed.
"We then analyzed this viral intervention in the cellular process in more detail to find out exactly which genes in the cells are affected," says Costa. "We were able to identify several candidates that have either antiviral or proviral properties."
Because certain immunomodulating drugs intervene precisely in these signaling pathways, there may be potential here for a therapeutic approach. This seems particularly promising because organ transplant recipients have to take immunosuppressive drugs for the rest of their lives to prevent the immune system from rejecting the transplant.
"However, this requires further studies," says Prof Ulrich Kalinke, Director of the Institute for Experimental Infection Research and Managing Director of TWINCORE.
More information: Bibiana Costa et al, Human cytomegalovirus exploits STING signaling and counteracts IFN/ISG induction to facilitate infection of dendritic cells, Nature Communications (2024). DOI: 10.1038/s41467-024-45614-3