This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Human stem cells coaxed to mimic the very early central nervous system
The first stem cell culture method that produces a full model of the early stages of the human central nervous system has been developed by a team of engineers and biologists at the University of Michigan, the Weizmann Institute of Science, and the University of Pennsylvania.
"Models like this will open doors for fundamental research to understand early development of the human central nervous system and how it could go wrong in different disorders," said Jianping Fu, U-M professor of mechanical engineering and corresponding author of the study in Nature.
The system is an example of a 3D human organoid—stem cell cultures that reflect key structural and functional properties of human organ systems but are partial or otherwise imperfect copies.
"We try to understand not only the basic biology of human brain development but also diseases—why we have brain-related diseases, their pathology, and how we can come up with effective strategies to treat them," said Guo-Li Ming, who along with Hongjun Song, both Perelman Professors of Neuroscience at UPenn and co-authors of the study, developed protocols for growing and guiding the cells and characterized the structural and cellular characteristics of the model.
For example, organoids developed using patient-derived stem cells may be used for identifying which drugs offer the most successful treatment.
Already, human brain and spinal cord organoids are used to study neurological and neuropsychiatric diseases, but they often mimic one part of the central nervous system and are disorganized. The new model, in contrast, recapitulates the development of all three sections of the embryonic brain and spinal cord simultaneously, a feat that has not been achieved in previous models.
"The system itself is really groundbreaking," said Orly Reiner, the Berstein-Mason Professorial Chair of Neurochemistry at Weizmann and co-author of the study who developed cellular tools to identify neural cell types in the model. "A model that mimics this structure and organization has not been done before, and it offers numerous possibilities for studying human brain development and especially developmental brain diseases."
While the model is faithful to many aspects of the early development of the brain and spinal cord, the team notes several important differences. For one, neural tube formation—the very first stage of central nervous system development—is very different. The model can't be used to simulate disorders that stem from improper closure of the neural tube, such as spina bifida.
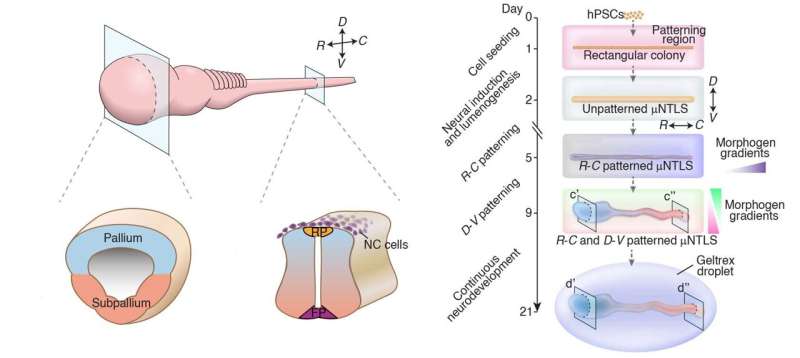
Instead, the model started with a row of stem cells roughly the size of the neural tube found in a 4-week-old embryo—about 4 millimeters long and 0.2 millimeters in width. The team stuck the cells to a chip patterned with tiny channels that the team used to introduce materials that enabled the stem cells to grow and guided them toward building a central nervous system.
The team then added a gel that allowed the cells to grow in three dimensions and chemical signals that nudged them to become the precursors of neural cells. In response, the cells formed a tubular structure. Next, the team introduced chemical signals that helped the cells identify where they were within the structure and progress to more specialized cell types.
As a result, the system organizes itself to mimic the forebrain, midbrain, hindbrain, and spinal cord in a way that mirrors embryonic development.
"As an engineer, the challenging part is to learn neural development and stem cell biology," said Xufeng Xue, the first author of the study and a postdoctoral fellow in mechanical engineering at U-M. "It was a team effort to make this happen, with amazing collaborators at UPenn and Weizmann."
The team grew the cells for 40 days, simulating the development of the central nervous system to about 11 weeks post-fertilization. In this time, the team was able to demonstrate the roles of specific genes in spinal cord development and learn how certain cell types in the early human nervous system differentiate into different cells with specialized functions.
"In many cases, animal models simply do not recapitulate either the characteristics or the degree of severity seen in human brain diseases such as microcephaly," Song said. "Even nonhuman primates are not the same. So in the context of disease biology and treatment strategies, a human cell model is almost irreplaceable."
The team plans to apply the model to study different human brain diseases using patient-derived stem cells.
Xue hopes to continue using this model to study the interplay among different parts of the brain during development. He is also interested in studying how the brain sends instructions for movement via the spinal cord. This line of inquiry, which could shed new light on disorders like paralysis, would require the neurons to link up into working circuits—something that was not observed in this study.
Insoo Hyun, a bioethicist at the Museum of Science in Boston who was not part of the study, notes that experiments like these are closely scrutinized before they are allowed to move forward.
"Research groups must be clear about the scientific question they are trying to answer—and that the degree of development they allow in the model is the minimum to answer the question," he said.
The model does not include peripheral nerves or functioning neural circuitry—features that are critical for humans' ability to experience our environment and process that experience.
More information: Xufeng Xue et al, A Patterned Human Neural Tube Model Using Microfluidic Gradients, Nature (2024). DOI: 10.1038/s41586-024-07204-7