This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New research seeks to determine whether cystic fibrosis can be treated in utero
Just five years ago, starting a family was almost unheard of for patients with cystic fibrosis (CF). And for expectant parents, it was often a huge shock when their child received a diagnosis of CF.
Affecting about 100,000 people worldwide, including 3,700 Australians, CF is an inherited disorder where one in 25 people carry the altered gene. When a person has two copies of that gene, the body's mucus and sweat become very thick and salty, severely damaging the lungs and digestive system.
As there is currently no cure for CF, people with the condition require intensive daily physiotherapy to clear the lungs and airways, countless medications and frequent hospitalizations.
Thankfully, the landscape of CF is now dramatically changing.
In the last 10 years, the predicted life expectancy of patients with CF has increased from 40 years of age to 71.6 years—if Trikafta drug therapy is started during adolescence.
The success of Trikafta and other medications also means more people with CF are becoming parents for the first time.
But as well as bringing joy, this creates a challenge. Because CF treatments are currently only approved for children over two years old, people are seeking much-needed guidance on how to proceed safely with these drugs during pregnancy and breastfeeding.
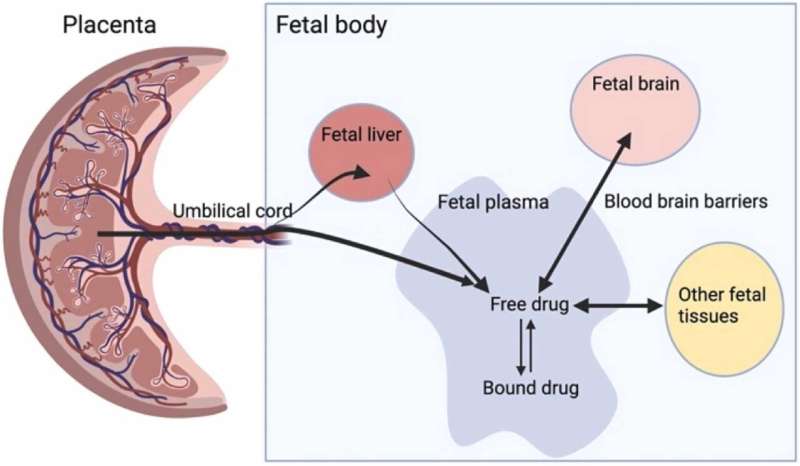
Our latest animal study, published in Biomedicine & Pharmacotherapy found that CF drugs cross the placenta and accumulate in fetal tissues.
We also showed for the first time that entry of Trikafta to the developing brain is not restricted by fetal blood-brain barriers (BBB)—a network of blood vessels and tissue that allows water and oxygen to pass into the brain but keeps out bacteria and some drugs. That study is published in the British Journal of Pharmacology.
To understand more about how Trikafta impacts human fetal development, we are now embarking on a study that includes women and babies—with and without CF—to better guide treatment.
Pregnancy and CF
Trifkafta is part of the highly effective modulator therapies (HEMTs), which correct the underlying genetic changes that cause CF disease, reducing effects on the lungs and digestive system.
In the clinical trials of Trikafta, pregnant women were excluded as it was expected that women with CF would not fall pregnant because of the effects of the condition and their shorter life expectancy.
However, this is not something we see in the clinic.
The number of pregnancies in the U.S. has doubled from around 300 in 2019 to almost 700 after the approval of Trikafta.
In Australia, Trikafta was added to the Pharmaceutical Benefits Scheme (PBS) in March 2022, and since then the number of pregnancies in mothers with CF has risen steadily to around 60 in 2023.
During this time, a handful of clinical reports from doctors also identified that all components of HEMT pass through the placenta.
So patients currently face a huge dilemma. If they stop drug treatment, their own lung function decreases again, putting them and their baby at higher risk for preterm delivery.
But Trikafta is currently only approved by the US Food and Drug Administration Food and Drug Administration (FDA) for use in children aged two and over.
So on the one hand, if a mother with CF takes Trikafta, the unborn child shouldn't be exposed to unnecessary drugs, as the effects are still unknown. On the other hand, an unborn child with CF might benefit significantly from early exposure, potentially preventing some of the irreversible symptoms like male infertility that begin before birth.
With 90 percent of CF patients estimated to be on HEMTs by 2025, families and clinicians urgently need solid information from larger studies to make informed decisions.
We need to avoid families feeling that their only option is to use CF medication outside current recommendations.
Developing better guidance for parents
Building on the individual clinical case reports, our latest research showed that when Trikafta is taken during pregnancy, it does cross the placenta in animal models, accumulating in key cystic fibrosis tissues including the pancreas, liver, lungs and the brain.
This is a significant finding, firstly because it highlights the need for long-term follow-up for babies with early exposure to Trikafta, but it also offers a promising new avenue of treatment. If safe, the accumulation of Trikafta in the baby's body could help to reduce the early effects of CF.
But, we are still missing larger data sets to provide the best guidance for patients.
With this in mind, our team has two major studies underway.
The first is in collaboration with Associate Professor Martin Donnelley's lab at the University of Adelaide to look at the benefits and potential risks of early life exposure to Trikafta.
This project will measure levels of Trikafta during pregnancy for both the mother and baby. Studying this in mothers with and without CF and their babies will give us a much-needed understanding of how Trikafta is absorbed and moves across the placenta.
We want to understand whether drug exposure early in life is safe for women with CF and their children, and whether it leads to prevention or delay in the onset of CF symptoms.
For the second study, we've partnered with Professor Jen Taylor-Cousar from the University of Colorado. Professor Taylor-Cousar leads the Maternal and Fetal Outcomes in the Era of Modulators (Mayflowers) trial—the first to evaluate changes in lung function in women with CF who are taking HEMTs during pregnancy and for two years after.
Closer to a cure
Our aim with this research is to add substantial knowledge on maternal and infant CF health, providing broad and long-term safety data for the use of Trikafta in pregnancy.
But importantly, the model adds another, even more, promising dimension: If Trikafta is safe during pregnancy, does early exposure to these drugs delay the onset of symptoms for the baby?
This could equate to something close to a cure.
Positive outcomes would represent a paradigm shift in the way HEMTs are prescribed during pregnancy and how cystic fibrosis—and potentially other diseases—are treated in utero.
Ultimately, we want to translate our findings into treatments that arrest the development and progression of CF symptoms, translating this knowledge into clinical guidelines.
Our end goal would be to change the labeling of Trikafta for pregnant women and children under 2 years of age.
Importantly, this research will also offer women living with cystic fibrosis a sense of normality—a normal pregnancy and all the wonderful experiences that it brings.
More information: Danni Li et al, Fetal drug exposure after maternally administered CFTR modulators Elexacaftor/Tezacaftor/Ivacaftor in a rat model, Biomedicine & Pharmacotherapy (2024). DOI: 10.1016/j.biopha.2024.116155
Danni Li et al, Extent of foetal exposure to maternal elexacaftor/tezacaftor/ivacaftor during pregnancy, British Journal of Pharmacology (2024). DOI: 10.1111/bph.16417