This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Multicancer early detection tests for cancer diagnosis
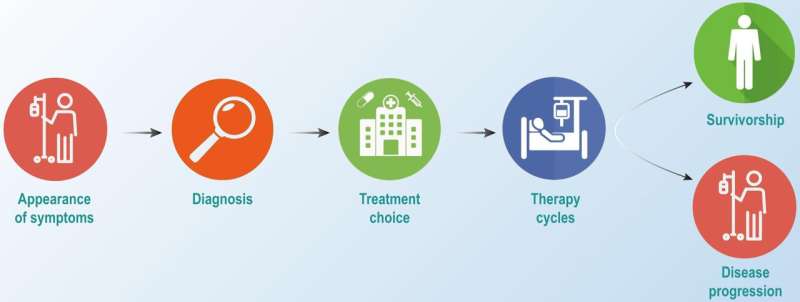
Cancer remains one of the most formidable public health challenges, causing significant mortality globally. In 2022 alone, there were approximately 19.3 million new cancer diagnoses and 10 million cancer-related deaths worldwide. The high mortality rate is primarily attributed to the late detection of the disease, often after it has metastasized, leaving limited treatment options. Early detection is crucial, as it could prevent at least 15% of cancer-related deaths over a five-year span by enabling the removal of precancerous lesions and the treatment of localized disease.
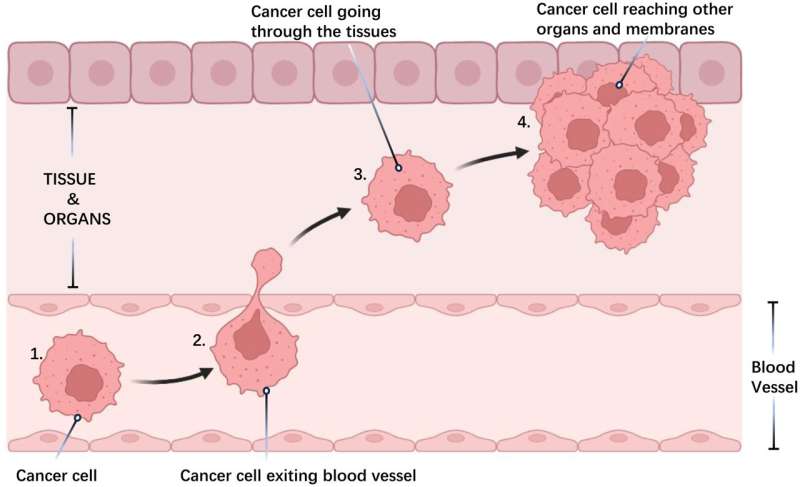
Cancer is characterized by the uncontrolled proliferation and spread of abnormal cells within the body. While normal cells undergo regulated growth and division, aging or damaged cells naturally die off and are replaced by new cells. However, when this process malfunctions, it can result in the formation of tumors, which can be either benign or malignant. Malignant tumors, unlike benign ones, invade nearby tissues and spread to other parts of the body through metastasis, which is responsible for the majority of cancer-related deaths.
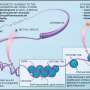
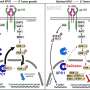
Recent advancements in cancer research have led to the development of multicancer early detection (MCED) tests. These tests represent a promising approach for identifying cancer at its earliest stages by analyzing tumor-related markers in bodily fluids, such as blood, and employing artificial intelligence to detect and differentiate various cancer types. MCED tests fall under the broader category of liquid biopsies, which are noninvasive and cost-effective alternatives to traditional tissue biopsies. They provide a comprehensive genomic snapshot of a tumor, allowing for the detection of specific biological signals in DNA, RNA, or proteins released by cancer cells.
A study on this topic appears in the Journal of Exploratory Research in Pharmacology.
MCED tests offer several advantages, including noninvasiveness, cost-effectiveness, and the ability to perform serial sampling for monitoring drug resistance and tumor progression. These tests detect fragments of DNA or RNA released by tumor cells into the bloodstream, helping to identify the most likely origin of the cancer. This capability is crucial for detecting early-stage cancers when they are most treatable.
Liquid biopsies, the foundation of MCED tests, have revolutionized the approach to cancer detection. Traditional biopsies, which involve the surgical removal of tissue, can be invasive, painful, and carry risks of complications. In contrast, liquid biopsies require only a blood sample, making the process far less invasive and more acceptable to patients. This method not only enhances patient comfort, but also allows for repeated sampling over time, enabling continuous monitoring of the cancer's progression or response to treatment.
Furthermore, liquid biopsies can capture the heterogeneity of tumors better than a single tissue biopsy, as they collect genetic information from cancer cells shed into the bloodstream from multiple sites within the body.
Despite their potential, MCED tests face significant challenges in clinical implementation, including the need for a standardized framework to assess their performance and safety. Currently, only a few MCED tests are available to doctors, and none have been approved by the Food and Drug Administration for market release. The specificity of these tests is generally high, but their sensitivity can vary depending on the type and stage of cancer.
The lack of standardized protocols for evaluating MCED tests poses a barrier to their widespread adoption. Each test employs different methodologies, biomarkers, and analytical techniques, making it difficult to compare results across studies or establish universal performance metrics. To address this, regulatory bodies and research institutions must collaborate to develop comprehensive guidelines that ensure the reliability and accuracy of MCED tests. This standardization is critical for gaining regulatory approval and integrating these tests into routine clinical practice.
MCED tests can be used both for symptomatic patients to minimize diagnostic delays and for screening seemingly healthy individuals to detect asymptomatic cancers. Liquid biopsies, which are the basis for MCED tests, have shown promise in clinical trials by providing a noninvasive means of detecting and monitoring cancer. The US Surveillance, Epidemiology, and End Results program has used state transition models to project the potential benefits of MCED tests, including diagnostic yield, stage shift, and reductions in mortality.
Several ongoing clinical trials are evaluating the efficacy of MCED tests. These studies are crucial for demonstrating the clinical utility of the tests, validating their ability to detect cancer early, and improving patient outcomes. Preliminary results from these trials have shown that MCED tests can detect multiple types of cancer with high specificity, although sensitivity varies. For example, trials have indicated that these tests are particularly effective in identifying cancers that are currently difficult to detect through conventional screening methods, such as pancreatic and ovarian cancers.
The development and implementation of MCED tests represent a significant advancement in the field of cancer detection and diagnosis. These tests have the potential to revolutionize cancer screening by enabling the early detection of multiple cancer types simultaneously. However, further research and standardization are needed to ensure their efficacy and safety before they can become a standard part of clinical practice. Continued innovation and investment in this area are essential for improving cancer survival rates and reducing the global burden of this disease.
More information: Kasturee Hajra et al, Multicancer Early Detection Tests for Cancer Diagnosis, Journal of Exploratory Research in Pharmacology (2024). DOI: 10.14218/JERP.2023.00007