This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Breaking down barriers: ROCK2 inhibition facilitates drug delivery in fibrotic pancreatic cancer
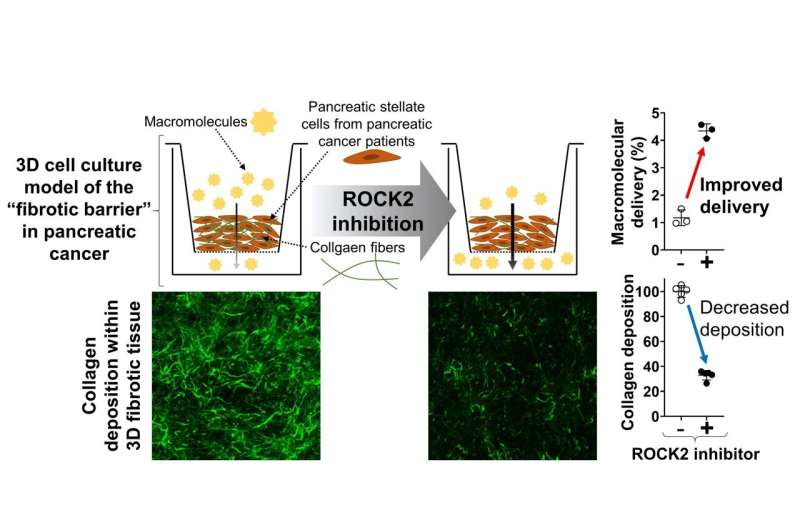
Pancreatic cancer, recognized as one of the deadliest cancers, poses a persistent challenge for medical professionals globally due to its aggressive behavior and resistance to conventional therapies. The dense fibrotic tissue surrounding pancreatic tumors acts as a significant barrier, hindering the delivery of macromolecular drugs such as antibodies and nanomedicines. Therefore, addressing fibrosis is crucial in enhancing therapeutic outcomes for patients with pancreatic cancer, whose prognosis remains bleak.
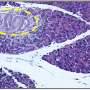
Understanding the underlying mechanisms driving fibrosis is essential in advancing treatment strategies for this devastating illness. The conventional models used for studying pancreatic ductal adenocarcinoma (PDAC) fibrotic environment often fail to accurately mimic the complexity of the disease.
Seeking a more clinically relevant approach, a team of researchers led by Professor Mitsunobu R. Kano from Okayama University, Japan in collaboration with The University of Tokyo and Tohoku University developed a realistic human pancreatic cancer fibrotic barrier model using patient-derived pancreatic stellate cells (PSCs). Their research, co-authored by Prof. Hiroyoshi Y. Tanaka from Okayama University, Prof. Horacio Cabral from The University of Tokyo, and Prof. Atsushi Masamune from Tohoku University, was published in the May 2024 issue of the Journal of Controlled Release.
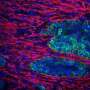
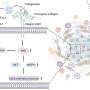
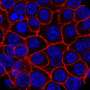
This innovative model offers a convenient, rapid, and highly reproducible experimental platform to assess pathogenetic mechanisms driving fibrosis as well as to test the efficacy of potential therapeutic interventions. Understanding the molecular mechanisms driving extracellular matrix (ECM) remodeling in PDAC fibrosis is crucial for overcoming treatment barriers.
By dissecting these signaling pathways, researchers aimed to determine points of intervention that could be manipulated to enhance tissue permeability and develop more effective treatments for pancreatic cancer.
"We used advanced cell culture techniques along with molecular and pharmacological interventions to examine the signaling pathways involved in PDAC fibrosis," explains Professor Kano. "Initially, we assessed the impact of blocking the transforming growth factor-β (TGFβ) and Rho-associated kinase (ROCK) pathways on ECM remodeling and tissue permeability within our 3D fibrotic tissue model.
"Additionally, we also investigated the involvement of YAP (Yes-associated protein), a key regulator of cell differentiation, in mediating the effects of ROCK inhibition on ECM remodeling and tissue permeability."
The results shed light on promising avenues for enhancing drug delivery in PDAC by targeting specific signaling pathways involved in fibrosis.
"Inhibition of the TGFβ pathway led to a reduction in ECM remodeling and improved tissue permeability of drugs in 3D-PDAC fibrotic model. Similarly, blocking the ROCK pathway, particularly through ROCK2 inhibition, resulted in decreased ECM remodeling and enhanced drug permeability. Knockdown experiments revealed the predominant role of ROCK2 in regulating these processes," says Prof. Kano.
"Additionally, targeting the YAP protein, a downstream effector of ROCK signaling, produced similar improvements in ECM remodeling and drug permeability." These findings highlight the potential of targeting the TGFβ/ROCK2/YAP signaling axis to mitigate fibrotic barriers and enhance drug delivery in pancreatic cancer, offering promising prospects for improving treatment outcomes in this challenging disease.
Looking ahead, the implications of the study extend beyond pancreatic cancer alone.
"Fibrosis is prevalent not only in pancreatic cancer but also in other cancers that are difficult to treat, such as those affecting the stomach, breast, and lung, as well as in some non-cancerous diseases. With effective medical treatments lacking for fibrotic conditions, our findings offer a glimmer of hope for enhancing treatment strategies and outcomes in a broader spectrum of fibrotic cancers as well as non-cancerous fibrotic conditions," concludes Prof. Kano.
More information: Hiroyoshi Y. Tanaka et al, Targeting ROCK2 improves macromolecular permeability in a 3D fibrotic pancreatic cancer microenvironment model, Journal of Controlled Release (2024). DOI: 10.1016/j.jconrel.2024.03.041