This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Breakthrough brain recording device receives FDA approval for a clinical trial
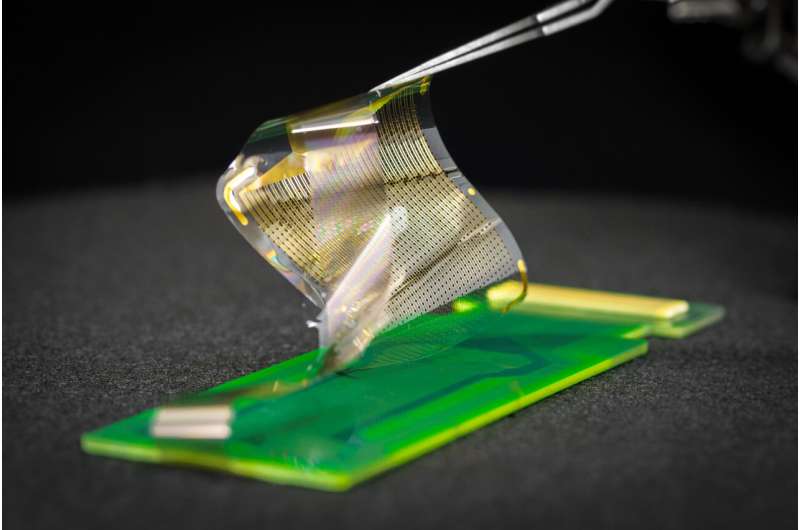
The Federal Drug Administration has approved a clinical trial to test the effectiveness of an electronic grid that records brain activity during surgery. The device was developed by engineers at the University of California San Diego.
The grid's nanoscale sensors record electrical signals directly from the surface of the human brain in record-breaking detail. The breakthrough resolution could provide better guidance for planning and performing surgeries to remove brain tumors and to treat drug-resistant epilepsy.
The grid's higher resolution for recording brain signals could improve neurosurgeons' ability to minimize damage to healthy brain tissue. During epilepsy surgery, the grid could improve the ability to precisely identify the regions of the brain where epileptic seizures originate for safe and effective treatment.
The new brain sensor array, known as platinum nanorod grid (PtNRGrid) features a densely packed grid of a record-breaking 1,024 embedded electrocorticography (ECoG) sensors. The device rests on the surface of the brain and is approximately 6 microns thin—smaller than one tenth of the human hair—and flexible.
As a result, it can both adhere and conform to the surface of the brain, bending as the brain moves while providing high-quality, high-resolution recordings of brain activity. In contrast, the ECoG grids most commonly used in surgeries today typically have between 16 and 64 sensors. These grids are rigid and more than 0.5 mm in thickness and do not conform to the curved surface of the brain.
The PtNRGrid was invented by Shadi Dayeh, a Professor in the Department of Electrical and Computer Engineering at the University of California San Diego and members of his team. Over the years, the team developed the PtNRGrid technology in collaboration with neurosurgeons and medical researchers from UC San Diego, Massachusetts General Hospital (MGH) and Oregon Health & Science University (OHSU).
"This accomplishment ushers in a new era of clinical neuroscience and neuromonitoring," Dayeh said. "We are very excited to receive the FDA approval to apply our groundbreaking PtNRGrid in a clinical setting. It is a credit to the hard work of my team members who worked tirelessly to meet the quality criteria mandated by the FDA.
"I am also grateful to my clinical partners, the support of the NIH, and to the campus leadership that fostered an impactful ecosystem across engineering and medicine to transform the future of health care."
The FDA approved an investigational device exemption (IDE) for a "pivotal study [titled] "Systematic Evaluation of Platinum Nanorod Grids (PtNRGrids) for Intraoperative Mapping and Neurophysiological Monitoring (IONM) During Brain Surgeries."
Specifically, the clinical trial is designed to demonstrate the effectiveness of the PtNRGrid device to map both normal and pathological brain activity. During the trial, UC San Diego engineers will partner with clinician-scientists Drs. Sharona Ben-Haim and Eric Halgren at UC San Diego, Dr. Sydney Cash at MGH, and Dr. Ahmed Raslan at OHSU.
In a first phase, surgeons will implant the PtNRGrid in 20 patients, then measure and compare the grid's performance with the present state-of-the-art. The PtNRGrid will be deployed in surgeries to remove brain tumors and to remove tissue that causes epileptic seizures.
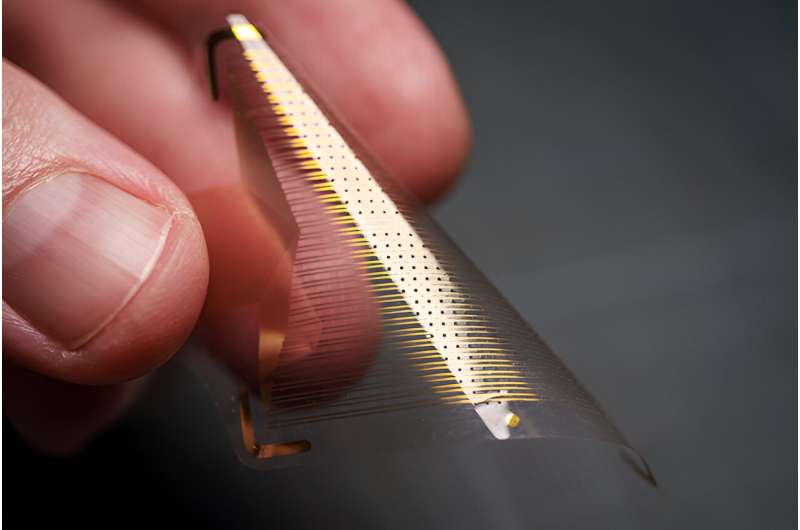
Record-breaking density
Dayeh's team has pioneered human brain and spinal cord mapping with thousands of channels since 2019, and has reported early safety and efficacy results in human subjects in a series of articles published in Science Translational Medicine in 2022.
PtNRGrid is the only device with thousands of channels to demonstrate in peer-reviewed publications that it can map motor and language brain activity, as well as epileptic discharges, by producing panoramic videos of brain waves over 10 square centimeters of the brain's cortex while maintaining resolution at a microscopic level.
Currently, Dayeh's research group holds the world record of recording brain activity from a single cortical grid with 2,048 channels on the surface of the human brain. This result was also published in Science Translational Medicine in 2022.
The device was used in the operating room of Dr. Ahmed Raslan of the OHSU. Since then, the team has increased the number of recording channels to 4,096 and continues to work on increasing the number of channels in the grid to monitor brain activity in even higher resolution.
Pending success of this staged trial, the team will transition to the next crucial step of making the PtNRGrid available for commercial use at scale. Demonstrating that ECoG grids with sensors in the thousands of channels record brain activity with high fidelity also opens new opportunities in neuroscience for uncovering a deeper understanding of how the human brain functions.
Basic science advances, in turn, could lead to improved treatments grounded in enhanced understanding of brain function.
"Our goal is to provide a new atlas for understanding and treating neurological disorders, working with a network of highly experienced clinical collaborators at UC San Diego, MGH, and OHSU," Dayeh said.