This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Cutting-edge imaging unravels sex-specific structural variations in the heart
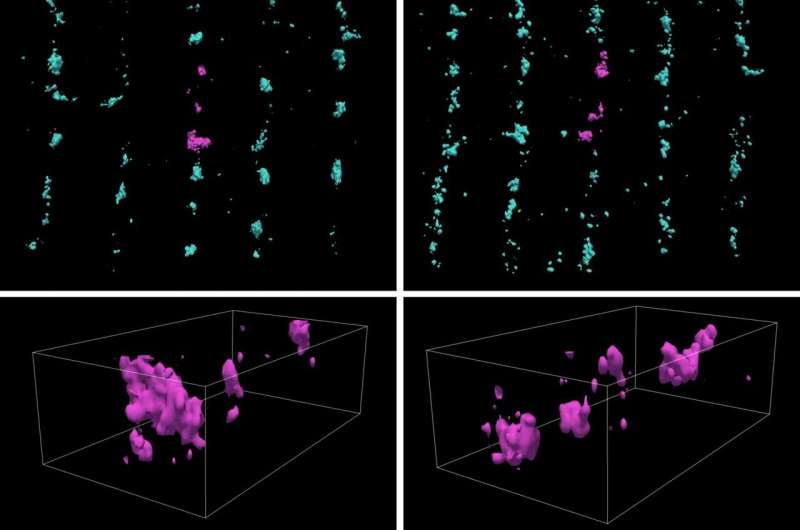
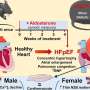
The hearts of men and women have remarkable differences in terms of their function in health and the heart diseases that impact them. In particular, there is a major difference between prevalent heart failure types experienced by men and women. Sex-specific approaches are very much needed to handle heart failure; we are at a stage where there are many gaps in our knowledge in relation to this topic.
A recent study published in the Journal of Physiology by the Laboratory of Systems Biology at TalTech (Tallinn, Estonia) with the collaboration of a group from Oslo University Hospital (Oslo, Norway) explores the unique traits of heart cells in men and women. The researchers employ cutting-edge microscopy techniques to explore the structural and functional disparities in the cardiomyocytes of male and female mice. Particularly, the researchers focus on the beat-to-beat calcium signals to uncover pivotal distinctions that may reshape our perspective on sex-specific heart physiology.
The heart is a unique organ that cannot take a break or rest. To function, it combines electrical stimulation, biochemical signaling and reactions with mechanical work. In this research, the groups looked at the link between electrical and biochemical signaling used in the heart in a single muscle cell. That biochemical signal is carried by calcium, which triggers mechanical work. Most previous studies on sex-specific differences in heart physiology suggest that male hearts display larger swings in calcium concentration due to a higher number of calcium releases from RyR (calcium sparks) and heightened stress responses during adrenergic stimulation.
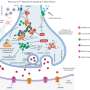
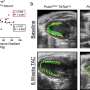
Many intracellular structures make this transition from electrical to chemical signals possible. These include specialized membrane structures (t-tubules) and calcium release channels (RyRs). In this project, the researchers focused on important calcium signaling-related intracellular structural elements, how their arrangement differs between sexes, and whether this impacts cardiac calcium signaling.
This study reveals unexpected subtleties. In contrast to earlier studies, this study shows that female heart cells showcase a higher frequency of elementary calcium release events—calcium sparks. The difference between earlier studies is likely caused by better conditioning of the cells before measurement. Leveraging cutting microscopy imaging techniques and advanced image analysis, the differences in calcium spark frequencies between female and male cardiac muscle cells are attributed to the differences in the arrangement of RyRs in female hearts. At the same time, there are differences in the arrangement of the t-tubular network between female and male cardiomyocytes.
However, delving further into the overall behavior of heart cells, the study identifies similar patterns in calcium concentration swings between men and women. Thus, these differences are balanced in intact cells, resulting in similar calcium signaling in electrically stimulated male and female cardiomyocytes. Nevertheless, the nuanced structural variations uncovered, along with the precision of advanced imaging, suggest potential insights into how heart pathologies may manifest differently in males and females. Based on these promising results, these advanced imaging techniques are further developed as part of Estonian Research Council project PSG832, which looks into the details of calcium signaling in the heart at the nanoscale.
In summary, the study sheds light on the subtle variations in calcium handling in male and female hearts. This not only deepens our understanding but also opens promising avenues for personalized sex-specific approaches to heart treatments.
More information: Martin Laasmaa et al, Cardiomyocytes from female compared to male mice have larger ryanodine receptor clusters and higher calcium spark frequency, The Journal of Physiology (2023). DOI: 10.1113/JP284515