This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
New study sheds light on the rate, nature and transmission of mitochondrial DNA mutations in humans
A new study from deCODE genetics uses pedigrees and sequence data from 64,806 Icelanders to shed light on the rate and nature of mutations in mitochondrial DNA (mtDNA) and the peculiar dynamics of its maternal transmission.
In a paper published in Cell, scientists from deCODE genetics, a subsidiary of Amgen, present the largest study to date of germline mtDNA mutations in humans and their transmission across 116,663 mother-child pairs.
The study documents the astonishing extent of hypermutability at some positions in mtDNA, including the well-known deleterious A>G mutation at position 3243 which causes the MELAS syndrome. The mutation occurred 15 times in the 2,548 matrilineal pedigrees, but typically disappeared after several generations, due to its severe impact on the health of carriers.
Strong aggregate evidence was uncovered for selection against many such short-lived deleterious mtDNA mutations in the pedigrees. The deCODE team also reported evidence for an extensive earlier episode of negative selection affecting mitochondria, called germline selection, where poorly functioning mitochondrial DNA molecules are discarded during the development of oocytes.
Finally, they used the large number of transmissions of mtDNA mutations in the pedigrees to reliably estimate that individuals inherit, on average, only around 3 units of mtDNA from their mothers—fewer than indicated by previous studies.
"It is remarkable that the hundreds of thousands of mtDNA carried by oocytes are derived from only about three of the mtDNA molecules originally carried by the mother," noted Agnar Helgason, a corresponding author of the paper.
"This drastic bottleneck determines the rapid rate at which new mutations in the mtDNA germline can become lost or fixed over just a handful of generations in a pedigree, and must be in part due to a selection process during the development of oocytes, where mtDNA molecules with deleterious mutations are removed from the germline."
"This study takes us a few steps closer to understanding the basis of the extraordinary variation in mutation rates across the nucleotides of the mtDNA genome, even between different alleles at the same position," said Kári Stefánsson, CEO of deCODE and a corresponding author of the paper.
"Unfortunately, it is the hypermutability of some pathogenic mutations that has made them so prevalent and thereby easier to discover. However, our findings suggest that many rarer pathogenic mtDNA mutations that are responsible for disease burden in human populations remain to be discovered."
Background information
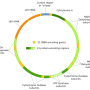
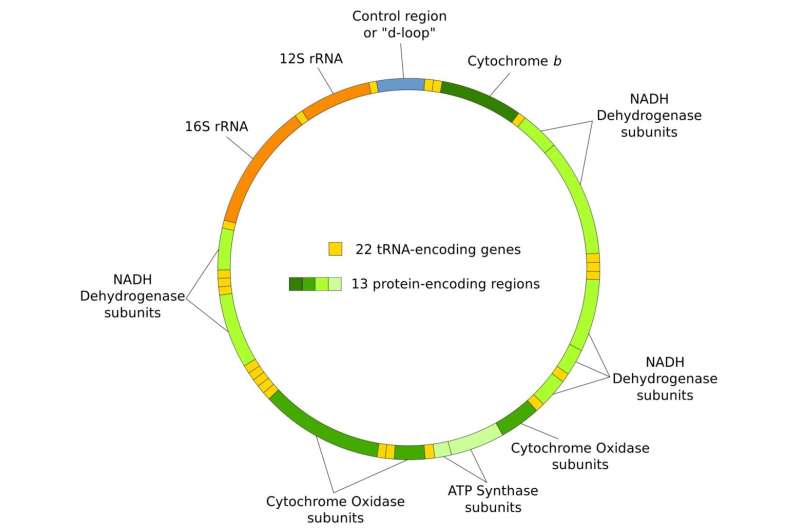
A small but important part of the human genome is found in mitochondria, cellular organelles that provide the energy that fuels many of the essential chemical reactions in cells. Mitochondrial DNA (mtDNA) is passed down only from mothers, via the oocyte (egg cell) that gives rise to each individual.
Each individual starts with hundreds of thousands of copies of mtDNA, derived from a small subset of the original mtDNA molecules carried by the mother—through a filtering mechanism known as the "germline bottleneck" when oocytes are produced.
When a sizeable proportion of the mtDNA copies transmitted from mother to child carry a deleterious mutation, the result is typically a particular combination of serious diseases, including stroke, type 2 diabetes, myopathies, blindness and deafness. Around one in every 5,000 individuals are affected by diseases due to known deleterious mtDNA mutations.
However, there are many more mutations whose deleterious impact has yet to be determined, leading to an expectation of an even greater disease burden due to mtDNA mutations.
Previous phylogenetic studies have shown that the germline mutation rate of mtDNA is around 20 times higher than that of the autosomal chromosomes. However, since such studies underestimate the mtDNA mutation rate, particularly for deleterious variants, a large study of mtDNA mutations in genealogies was needed to gain a more comprehensive understanding of their rate and nature and impact on human health.
The deCODE study used sequence data from 64,806 Icelanders, arranged into an extensive set of 2,548 matrilineal genealogies (matrilines), spanning 116,663 mother-child transmissions of mtDNA, to detect 8,199 mutations. The largest matriline contained 2,056 transmissions, linking 1,051 sequenced individuals traced back to an ancestor born around 1520.
The mutation rate estimates are 2.87×10-6 and 2.38×10-5 mutations per position per generation for the coding and control regions of mtDNA, respectively, around five-times greater than previous phylogenetic estimates.
A key novelty leading from such an extensive set of mtDNA mutations identified in matrilines is the possibility of distinguishing between two kinds of selection against deleterious mutations.
First, by comparing them with mutations ascertained from a phylogeny, they were able to assess the strength of selection that removes deleterious, but viable variants from populations over many generations.
Evidence of hypermutability was observed for some well-known deleterious mtDNA mutations, including the A>G mutation at position 3243, which occurred 15 times in the matrilines, but typically disappears after several generations, due to its severe impact on the health of carriers.
Second, there was strong evidence for extensive negative selection that occurs before individuals are conceived or born, thereby affecting the kinds of mutations that can be detected in live-born humans.
The most likely mechanism is germline selection, whereby poorly functioning mitochondrial DNA molecules are discarded during the development of oocytes. This would reduce the size of the germline bottleneck, that determines the number of mtDNA molecules transmitted from mothers to offspring during the development of oocytes.
Indeed, using the large number of transmissions of mtDNA mutations in the Icelandic matrilines, the deCODE team were able to reliably estimate that individuals inherit, on average, only around 3 units of mtDNA from their mothers.
More information: The rate and nature of mitochondrial DNA mutations in human pedigrees, Cell (2024). DOI: 10.1016/j.cell.2024.05.022. www.cell.com/cell/fulltext/S0092-8674(24)00531-2