This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unveiling Telo-seq: New method for determining the length and sequence of telomeres on individual chromosomes
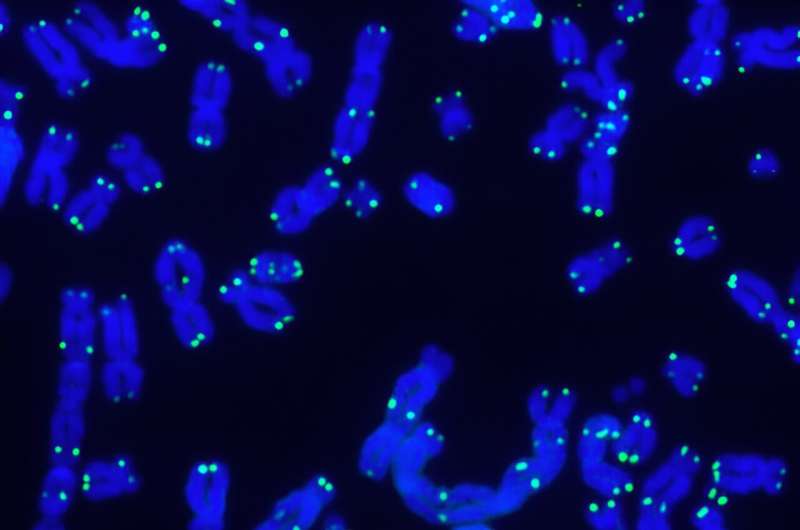
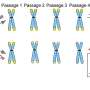
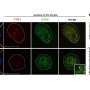
Within each of our cells, long strands of DNA are folded into chromosomes and capped with protective structures called telomeres. But telomeres shorten as we age, eventually getting so whittled down that our chromosomes become exposed, and our cells die. However, the specifics of when and how this shortening occurs and whether certain chromosomes are more affected than others have been unclear—until now.
Scientists at the Salk Institute have developed a tool called Telo-seq, designed to revolutionize the study of telomeres in aging and disease.
Compared to existing methods, which struggle to sequence whole telomeres and can only measure their average length across all chromosomes, the new technique allows researchers to determine the entire sequence and precise length of telomeres on each individual chromosome.
The researchers are already using Telo-seq to reveal novel telomere dynamics in human health and disease with unprecedented resolution. The findings, published in Nature Communications on June 18, 2024, will inspire a flurry of new studies and telomere-targeting therapeutics to treat age-related diseases.
"Previous methods for measuring telomere length were low resolution and rather inaccurate," says the study's senior author Jan Karlseder, professor, chief science officer, and Donald and Darlene Shiley Chair for Research on Aging at Salk.
"We could hypothesize about how individual telomeres might play a role in aging and cancer, but it was simply impossible to test those hypotheses. Now we can."
Karlseder and colleagues collaborated closely with experts at Oxford Nanopore Technologies to combine aspects of their long-read sequencing technique with novel biochemistry and bioinformatics approaches.
The resulting method starts at the end of each telomere and sequences well into the subtelomere region. This allows the scientists to identify which chromosome they are looking at and examine its telomere structure and composition in detail.
Using this technique, the researchers have described numerous features of telomere biology that had not been accessible to scientists before. So far, they've observed that within individual human samples, each chromosome arm can have different telomere lengths, and these telomeres can vary significantly in their shortening rates.
These dynamics vary in different tissues and cell types within the same person, likely for many reasons including the amount of stress and inflammation affecting different parts of the body. Altogether, this suggests that there are potential chromosome arm-specific factors influencing telomere dynamics in aging and disease.
"Aging is an incredibly heterogeneous process that affects everyone differently," says Karlseder. "We are very interested in whether differences in aging are related to different telomere shortening rates between people or chromosomes, and how we might be able to slow this down to promote healthy aging."
Telo-seq can also improve our understanding of telomere-driven diseases. Many telomeropathies involve stem cells that run out of telomere length and lose their ability to divide into new, functional cells. This can lead to hair loss, immune disorders, or certain cancers.
Telo-seq will allow scientists to investigate whether these diseases are inherited within families or associated with individual chromosomes, in order to develop more targeted interventions.
While telomere shortening can have devastating effects on a cell's lifespan, the opposite scenario can be equally damaging. When telomere repair mechanisms are overactivated, cells can enter an "immortal" state and divide indefinitely, leading to cancer.
To repair a damaged telomere, cells can either use the telomerase enzyme or another mechanism known as the alternative lengthening of telomeres (ALT). The length and composition of the telomeres will differ depending on which maintenance mechanism was used but, until now, there was no efficient way for scientists or clinicians to measure this.
"With Telo-seq, we can quickly determine whether a cancer is telomerase-positive or ALT-positive," says first author Tobias Schmidt, a postdoctoral researcher in Karlseder's lab.
"This is critical because ALT-positive cancers are often more aggressive and require different treatment approaches than telomerase-positive cancers. In this sense, Telo-seq could be used as a quick and reliable diagnostic tool to identify cancer types and guide more personalized treatment plans."
Beyond its many immediate clinical applications, Karlseder and Schmidt say Telo-seq's greatest impact will be in igniting a new era of telomere research.
"Telo-seq will allow us to answer questions about development, aging, stem cells, and cancer that we simply couldn't address with previous tools," says Karsleder.
"We don't even know what we've been missing, and I think the things we're starting to learn now are really just the tip of the iceberg. It's a very exciting time for telomere science."
More information: Tobias T. Schmidt et al, High resolution long-read telomere sequencing reveals dynamic mechanisms in aging and cancer, Nature Communications (2024). DOI: 10.1038/s41467-024-48917-7