This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Protein-based homing device precisely delivers drugs to target neurons
Biomedical engineers at Duke University have developed a method to deliver drugs to specific types of neurons throughout the brain. This new approach, which was tested in mice, was 100 times more precise than existing methods and enables researchers to more effectively study neurological diseases and explore efficient, targeted drug treatments.
The research was published June 14 in the journal Nature Methods.
Physicians and researchers have long relied on drugs to both study the complex connections between neurons and treat neurological diseases. But these drugs also lack a key characteristic to fully accomplish these goals––precision.
Drugs are designed to bind to specific receptors to trigger a response, but there can be millions of cells that express the same receptor. This means that when someone takes a drug, it affects all of those cells at the same time, triggering off-target side effects and making it difficult for researchers to identify the key cell signals impacted by the pharmaceutical.
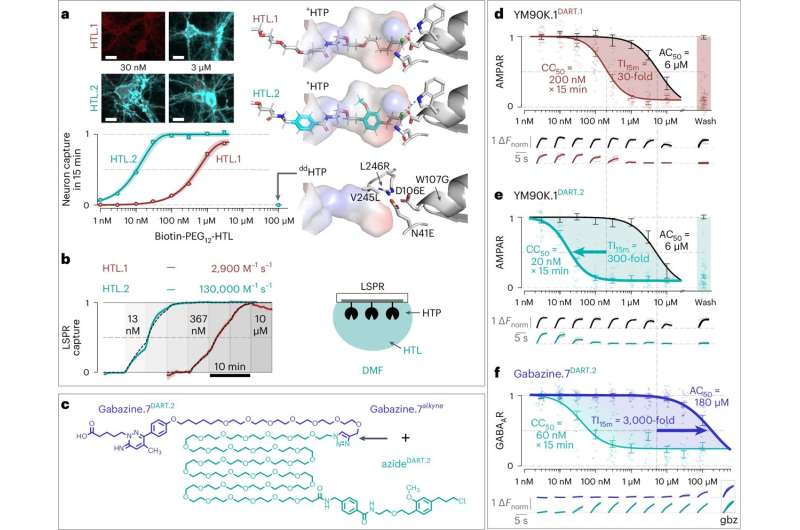
Michael Tadross and his collaborators sought to remedy this issue with the creation of DART, or Drug Acutely Restricted by Tethering. The first iteration of this tool allowed researchers to deliver pharmaceuticals to specific cell types within the brain by introducing a protein that coats their surfaces and attracts drugs loaded with a special homing device.
When a drug is introduced into the system, it's captured by this beacon and creates a locally high concentration of the drug around the targeted cells.
In the original iteration, the homing system allowed drugs to locally accumulate onto desired cells, reaching concentrations approximately 30 times higher than anywhere else in the brain within minutes. This worked well in most cases, however it wasn't a general solution.
"Think of the drug molecules like shoppers at a store that you'd like to purchase a specific item. Some of the shoppers may get there quickly, but others will get distracted by other items around the store and cause some mayhem. Just like these shoppers, our drugs can have unintended effects on their way to their target," says Tadross, assistant professor of biomedical engineering.
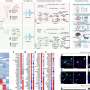
Now, Tadross and his colleagues have introduced DART.2, the second iteration of their tool. Boasting an optimized drug capture system, this version is 100 times more precise than its predecessor, allowing target cells to capture drugs at concentrations up to 3,000 times higher than anywhere else in the brain in just 15 minutes.
This improved precision will allow researchers to use drugs in live animals that were previously impossible. As a proof-of-concept, the researchers experimented with gabazine, a drug that prevents the neurotransmitter GABA (gamma-aminobutryic acid) from binding to GABA receptors.
"We tried to use gabazine with our initial DART tool, but it wasn't efficient enough," said Tadross. "Our optimized system solved this, making it possible to safely deliver gabazine to the targeted neurons without triggering an off-target epileptic response."
As an inhibitory neurotransmitter, GABA limits the ability of a neuron to send and receive signals, producing a calming effect. But by preventing GABA from binding to its receptors, gabazine can increase neural activity.
This has been an essential tool, helping researchers study GABA receptors in highly controlled and limited experiments, such as in cell culture or in a brain slice. But before now, it was very difficult to use in live animals, as even low doses can trigger seizures.
In a show of DART.2's capabilities, the team used gabazine to explore the role of GABA receptors in the ventral tegmental area, a brain region associated with behaviors like addiction, stress control, memory, and movement.
Previous studies suggested that GABA receptors in this brain region act like a gas pedal, making mice more active. Surprisingly, DART.2 showed the opposite was true for GABA receptors on dopamine neurons––GABA receptors on these cells served as a brake pedal, causing mice to slow down as if taking time to deliberate on their actions.
The increased precision of DART.2 also allowed the researchers to use a simpler route of administration. In earlier work, the team used a cannula to apply the drug close to its intended target. But with the new version of DART, this is no longer necessary.
They demonstrated delivery via the cerebrospinal fluid, allowing drug to circulate throughout the entire brain. The DART.2 system works efficiently enough to concentrate drug onto the specific brain region and cells of interest, while the rest of the brain experiences negligible off-target effects.
"Now the analogy becomes like a Black Friday sale where the items of interest have signs that lead everyone directly to the sale items," said Tadross. "The same was true when we delivered the drugs to the entire brain."
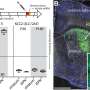
Inspired by best practices in clinical trials, the team also developed methods to confirm target engagement. They did this with fluorescent tracers that can be mixed and matched with any drug, enabling researchers to visualize where and how much of the drug was delivered.
As a final test for the updated tool, the team showed that DART.2 could precisely deliver drugs to both inhibit and strengthen the two major forms of synaptic transmission in the brain. Thus, they not only blocked GABA receptors, but could make them more sensitive to GABA.
They also extended the approach to AMPA receptors, which sense glutamate, an excitatory neurotransmitter that carries the majority of information transfer between neurons in the brain.
Tadross and his collaborators already have ideas for the next iteration of DART. One involves developing a system that is capable of crossing the blood-brain barrier. They'd also like to develop a new tracer that enables to them to track the location and concentration of drugs by using noninvasive imaging in live animals.
"Our first study with DART in 2017 was a proof of concept," said Tadross. "The idea was deceptively simple––we wanted a drug to work one place and not work another place, and we did that by controlling the drug concentration.
"Now, by extending the approach to more drugs and showing how drastically we can optimize the system, we're taking that next step. It's very exciting."
More information: Brenda C. Shields et al, DART.2: bidirectional synaptic pharmacology with thousandfold cellular specificity, Nature Methods (2024). DOI: 10.1038/s41592-024-02292-9