This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Study: Involvement of TAL1-microRNA axis in the progression of T-cell acute lymphoblastic leukemia
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive form of leukemia that arises from the malignant transformation of T-cell progenitors. This disease is most commonly diagnosed in children, where it accounts for a significant portion of pediatric leukemia cases, but it also affects adults. The clinical presentation of T-ALL includes symptoms resulting from bone marrow failure, such as anemia, thrombocytopenia, and neutropenia, as well as symptoms due to extramedullary disease, including lymphadenopathy, splenomegaly, and mediastinal masses.
Despite improvements in treatment protocols, which have led to higher remission rates, the prognosis for T-ALL remains challenging due to high rates of relapse, particularly in adult patients.
T-ALL pathogenesis involves a series of genetic and epigenetic changes that disrupt normal T-cell development and lead to uncontrolled proliferation. Central to these changes are alterations in the expression and function of key transcription factors that regulate T-cell differentiation and proliferation.
One such transcription factor is TAL1 (T-cell acute lymphoblastic leukemia 1), also known as SCL (stem cell leukemia). TAL1 is essential for the development of hematopoietic cells and is aberrantly expressed in a significant subset of T-ALL cases. The inappropriate expression of TAL1 in T-cell progenitors leads to a block in differentiation and promotes leukemogenesis.
TAL1 is normally involved in early hematopoiesis and is crucial for the development of erythroid and megakaryocytic lineages. In the context of T-ALL, however, TAL1 expression is frequently dysregulated due to chromosomal rearrangements or mutations. This ectopic expression of TAL1 interferes with the normal transcriptional programs of T-cells and leads to leukemic transformation. TAL1 exerts its oncogenic effects by altering the expression of a variety of target genes, including those involved in cell cycle regulation, apoptosis, and cell adhesion.
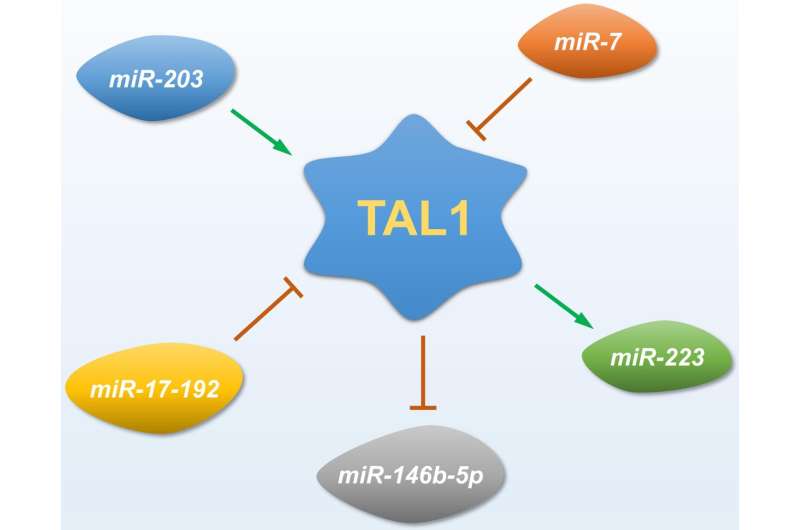
One of the key mechanisms through which TAL1 promotes T-ALL is by modulating the expression of microRNAs (miRNAs). MiRNAs are small non-coding RNAs that regulate gene expression post-transcriptionally and play crucial roles in various cellular processes, including differentiation, proliferation, and apoptosis. In T-ALL, TAL1 has been shown to downregulate specific miRNAs that act as tumor suppressors. Among these, miR-146b-5p has emerged as a critical player.
MiR-146b-5p is a tumor suppressor miRNA that is highly expressed in normal single-positive thymocytes. It regulates the expression of genes involved in cell migration and invasion, processes that are crucial for the metastatic potential of cancer cells.
In T-ALL, the downregulation of miR-146b-5p by TAL1 leads to the upregulation of its target genes, which include those involved in cell motility and invasion. This dysregulation enhances the invasive properties of T-ALL cells, facilitating their spread to various tissues.
The loss of miR-146b-5p expression has been associated with increased aggressiveness of T-ALL and poor prognosis. By targeting the TAL1-miR-146b-5p axis, researchers aim to develop new therapeutic strategies that can limit the spread of leukemia cells and improve patient outcomes. Restoring the expression of miR-146b-5p or inhibiting the pathways activated by its loss could potentially reduce the invasive and migratory capabilities of T-ALL cells.
The development and progression of T-ALL involves a complex interplay of genetic and epigenetic factors. The TAL1 transcription factor plays a central role in this process by dysregulating the expression of key genes and miRNAs, including the tumor suppressor miR-146b-5p. Understanding the molecular mechanisms underlying TAL1-mediated leukemogenesis provides valuable insights into the pathogenesis of T-ALL and identifies potential targets for therapeutic intervention.
Future research should focus on elucidating the upstream regulatory mechanisms that control TAL1 expression and its downstream targets, as well as exploring the therapeutic potential of modulating the TAL1-miR-146b-5p axis. Such efforts could lead to the development of novel treatments that improve the prognosis for patients with this aggressive form of leukemia.
The study is published in the journal Gene Expression.
More information: Daryush Purrahman et al, Involvement of TAL1-microRNA Axis in the Progression of T-cell Acute Lymphoblastic Leukemia, Gene Expression (2024). DOI: 10.14218/GE.2023.00076