This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Preclinical model offers new insights into Parkinson's disease process
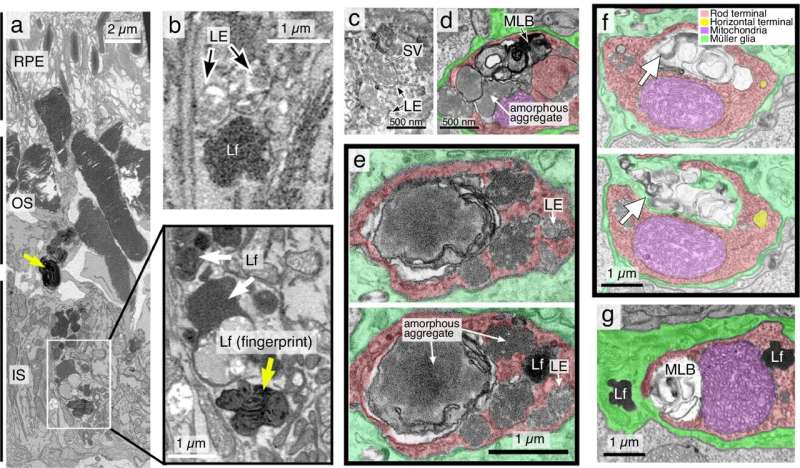
A new preclinical model offers a unique platform for studying the Parkinson's disease process and suggests a relatively easy method for detecting the disease in people, according to a new study led by Weill Cornell Medicine researchers.
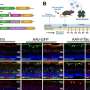
In the study, published July 23 in Nature Communications, the researchers show that knocking out a key component involved in protein transportation in the light-sensing rod cells of mice leads to the retinal accumulation of the aggregates of a protein called alpha-synuclein found in patients with Parkinson's disease.
"This is a really unique model involving a pathology that seems more like human Parkinson's than what we see in other mouse models," said Dr. Ching-Hwa Sung, the Betty Neuwirth Lee and Chilly Professor of Stem Cell Research and a professor of cell biology in ophthalmology and of cell and developmental biology at Weill Cornell Medicine.
The study's first authors were Drs. Cheng Fu, Nan Yang, Nobuyuki Nakajima, and Satoshi Iraha, all postdoctoral researchers in the Sung Laboratory during the study, and Dr. Jen-Zen Chuang, associate professor of cell biology research in ophthalmology at Weill Cornell Medicine. Dr. Neeta Roy, assistant professor of neuroscience research in ophthalmology at Weill Cornell Medicine, also contributed to the study.
Parkinson's disease, the second most common neurodegenerative disease after Alzheimer's, affects approximately one million Americans, and is diagnosed in the United States at the rate of about 90,000 new cases annually. Although it is popularly known as a movement disorder, its effects on the brain and body are widespread and can include early vision problems, dementia, sleep disorders, and reduced intestinal function.
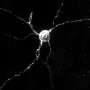
For the study, the researchers engineered mice that lack the gene for a protein called VPS35 just in rod cells, the main light-sensing neurons of the retina. VPS35 is known for helping cells to distribute molecules to their corresponding destinations, including sending abnormal proteins for degradation. A mutation in VPS35's gene has been linked to a familial form of Parkinson's disease.
The researchers observed that even in young mice, the rods lacking VPS35 soon lost their synapses—connection points to other neurons—resulting in visual impairment similar to those seen in patients with Parkinson's.
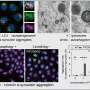
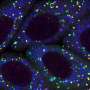
Alpha-synuclein aggregates began to form, and eventually, as the affected rods began to die, the mouse retinas showed large, insoluble inclusions that looked like Lewy bodies, which contain alpha-synuclein aggregates and are one of the classic pathological signs of Parkinson's disease.
As part of the study, the researchers traced VPS35's interactions with other proteins and found evidence that it works not just in disposing of aggregated alpha-synuclein but also in preventing its aggregation.
"We think this explains why we saw such a strong effect of knocking out this protein," Dr. Sung said.
The results suggest that the new model could be very useful for studying disease mechanisms and testing potential therapies, she added. The model's advantages include a rapidly developing disease process, and the absence of any artificial modification to the mice's alpha-synuclein—unlike existing models that drive pathology using excess, mutant or non-mouse forms of the protein.
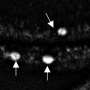
The findings with the new model also point to a potential new strategy for detecting Parkinson's disease. Even in three-month old mice lacking rod-cell VPS35, the researchers could use a standard ophthalmological device called a fundoscope to observe bright spots of "autofluorescence" caused by molecules called lipofuscin, which associate with alpha-synuclein aggregates. Dr. Sung and her international physician collaborators are currently planning a clinical trial of this approach.
Dr. Sung also plans to investigate the use of VPS35-knockout mice for studying Alzheimer's disease, since VPS35 mutations have been associated with that disease as well.
"This is the beginning of what we expect to be a very interesting exploration," Dr. Sung said.
More information: Cheng Fu et al, Mutant mice with rod-specific VPS35 deletion exhibit retinal α-synuclein pathology-associated degeneration, Nature Communications (2024). DOI: 10.1038/s41467-024-50189-0