This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Q&A: How a stem cell bank is helping scientists understand psychiatric disorders
When Ralda Nehme, a cell biologist and neuroscientist, first started her lab at the Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard in 2018, she realized a gap in the field. She was adept at growing stem cells in the lab, converting them into neurons, and using those cells to study the effects of genetic mutations linked to schizophrenia.
But she soon realized that to truly capture the complexity of human disease, she would need to study a large number of cells from many people with or without the disease and with different genetic backgrounds.
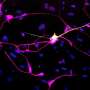
To meet this goal, Nehme and her lab established the Stanley Center's Stem Cell Resource. Blood or skin cells from donors can be treated with special proteins to turn them into induced pluripotent stem cells (iPSCs), which Nehme's team then differentiates into any cell type in the human body, all bearing the donor's genetic makeup, including any disease-causing gene variants.
Currently, the resource holds frozen cell lines from about 1,000 donors with a range of diagnoses and ancestral backgrounds, which scientists can use to generate different cell types that more faithfully model human disease than animal cell lines.
We spoke with Nehme about why these models are particularly useful for studying psychiatric conditions, important considerations for new cell lines, and her hopes for the future in this Q&A.
Why are iPSCs useful for studying psychiatric conditions?
For psychiatric disorders, having access to living human cells that we can manipulate in the dish is critical. The human component is important because we need to take the genetic landscape into consideration. In a mouse, we can manipulate the expression of a specific gene, but we typically don't manipulate the expression of hundreds or thousands of genes at once.
But human cells include that genetic background, which can really influence disease. And while human brain tissue is valuable, we often have limited access to postmortem brain tissue during specific stages of development, and we can't treat postmortem tissue with drugs or genetic perturbations and study how cells respond.
Of course, stem cells aren't perfect and there are artifacts due to culturing. People say, "All models are wrong, but some are useful." It's very true.
What kinds of questions is your lab trying to answer with these cells?
We have many different ongoing studies looking at how cells from different people respond to pharmacological perturbations such as antipsychotic medications. We know that different people respond in different ways to the same drug, but we don't always know why.
In the lab, we can treat astrocytes and neurons from patients with schizophrenia with different medications and see how the cells respond at the molecular level. We're beginning to see some really interesting differences in the cells after certain perturbations.
In a collaboration with Anne Carpenter and Soumya Raychaudhuri, we looked at cell morphology across iPS cell lines derived from roughly 300 people, and we were able to identify cell morphology traits that are associated with specific genetic variants.
In a follow-up study, we're applying a similar approach to neurons, astrocytes, and neural progenitor cells to identify in an unbiased way how cell morphology is affected by the presence of specific genetic variants.
The possible applications are almost limitless. The more data types we generate and integrate across different labs, the more powerful a resource it's going to be.
Why would a researcher want to study a disease process in cells?
We want to perform biological experiments at a scale that is sufficient to generate enough data to define relationships such as which genes cause disease in a statistically significant way. One way we can do that is to study thousands of cell lines at a reasonable cost and within a reasonable timeframe—which is much harder to do in animal models.
In collaboration with Steve McCarroll, we developed "cell village" experimental systems where we can mix cells from many different people together all in one dish and treat them with a certain agent. Then we use the cells' DNA to identify the donor of each cell. If you wanted to study cells from 100 people, instead of having 100 dishes in the incubator, we'd have just one.
Are there any cell types that you're particularly excited about being able to generate?
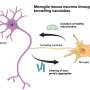
Astrocytes are a cell type that is abundant in the brain and has many different functions. They interact with neurons and a lot of genes that are critical for these interactions have been implicated in many psychiatric neurodevelopmental and neurodegenerative disorders.
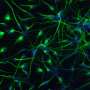
So in a collaboration with Lindy Barrett's lab in the Stanley Center, we developed a way to make astrocytes that is very scalable. We can now make them in a month. It used to take six. We can even grow them together with human neurons.
For a while, the whole field was using rodent astrocytes in co-culture with the human neurons, but human neurons really need the presence of the astrocytes to become functional. Having found a way around this is going to enable a lot of exciting approaches where we can manipulate genes and cellular programs in astrocytes and ask what the effect is on neurons. Being able to manipulate this biology in a disease-relevant context is really helpful.
Why is genetic diversity in these cells so important?
First, it's important to study not just cells from males but also females. For a while, a lot of people in the stem cell field were only focusing on using cells that are derived from white males to make the cohort more homogeneous. But when we focus on white males, we then miss a big chunk of biology.
Second, to drive scientific discovery, it's critical to capture as many variants as possible in different genes. Some variants are represented in a different way in different ancestral populations; some are not present at all in one population, but are in another. We also know that genetic ancestry can affect how well we can make stem cells from different populations by impacting differentiation and other cellular phenotypes.
Finally, we know that most clinical trials are based in the U.S. or in Europe. As a result, drug safety and toxicity studies are often tailored for populations in the U.S. and in Europe, and populations outside of these continents are often not as thoroughly considered.
It's impossible to run clinical trials for every drug in many different countries for cost reasons, but it's possible to take cells from any person anywhere in the world, make stem cells, and then try to see how they respond to drugs. That could be a game changer for many of these medications and populations down the road.
What do you hope to see from the field in the next 10 years?
We need better models of psychiatric conditions that are informed from the large amount of data we now have from profiling postmortem brains, for example, that can inform the next generation of better stem cell-based models with increased fidelity to in vivo profiles.
To do that, we need access to more cell lines from different populations that are quality controlled, cataloged, and accessible to the community. I think there's a really important role from funding agencies to encourage the use of many different cell lines and fund this kind of work, which is expensive and impossible for many labs.
But I also think we can lower the bar and make this kind of work more accessible. Realistically, it's hard for many labs to work with a hundred cell lines at a time. But we can build stem cell villages and freeze them, and then people can then just work with one vial. The work is very similar to working with just one cell line, except that they'll have access to a hundred cell lines.
We work with cells from so many people, including a lot of patients that are affected with these debilitating disorders and their families. It's an incredible privilege to work with this resource, and I feel very fortunate to be able to help researchers use it to ask interesting questions.