This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemical screen identifies PRMT5 as therapeutic target for paclitaxel-resistant triple-negative breast cancer
In a study published in Cell Chemical Biology, a research team led by Prof. Tan Weihong and Prof. Wu Qin from the Hangzhou Institute of Medical of the Chinese Academy of Sciences has identified protein arginine methyltransferases (PRMTs), crucial regulators of RNA splicing and chromatin stability, as a new therapeutic target for overcoming paclitaxel resistance in triple-negative breast cancer (TNBC).
Researchers discovered a comprehensive screen of an epigenetic small molecule inhibitor library. Based on previous research by Prof. Wu's team, which explored the role of RNA splicing in TNBC immune evasion, they unveiled a novel approach to combat drug resistance in TNBC by targeting the epigenetic mechanisms driving tumor growth.
TNBC is notoriously difficult to treat due to its lack of estrogen receptors, progesterone receptors, and HER2 overexpression. While paclitaxel is initially effective for many patients, resistance to the drug commonly develops within five to seven months, leading to disease progression and limited treatment options.
This study highlights the role of epigenetic modifications in promoting paclitaxel resistance, particularly through chromatin-mediated repression of transposable elements, which silences the viral mimicry response and supports tumor survival.
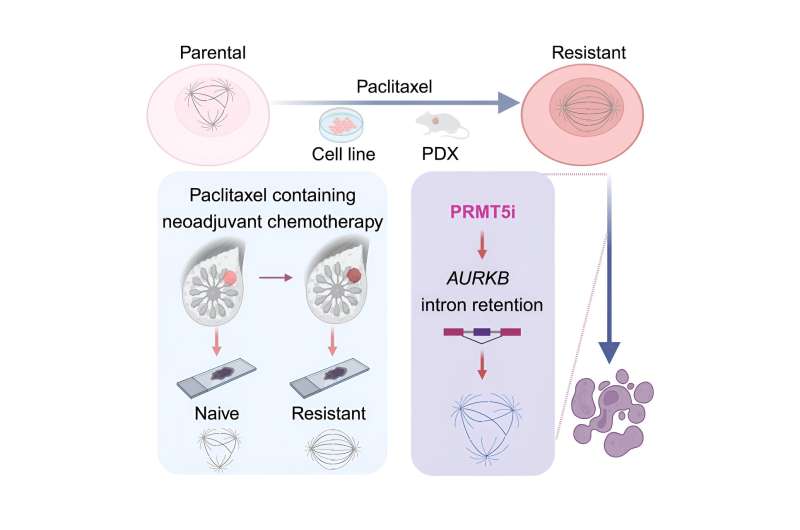
Researchers generated three distinct paclitaxel-resistant TNBC cell lines and, through extensive screening, identified a heightened sensitivity of these resistant cells to PRMT5 inhibitors.
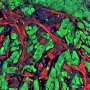
Using a combination of cellular biology and bioinformatics techniques, they discovered that PRMT inhibition impairs chromosomal stability and disrupts RNA splicing, notably affecting the mitotic checkpoint regulator AURKB. By inducing intron retention within the AURKB gene, PRMT inhibitors prevent the proper translation of this critical protein, thereby reducing chromosomal stability and triggering cell apoptosis.
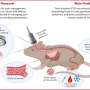
The therapeutic potential of PRMT inhibition was validated in both patient-derived xenograft (PDX) models and clinical samples. In addition, it was demonstrated that combining PRMT5 inhibitors with type I PRMT inhibitors synergistically suppresses the growth of paclitaxel-resistant TNBC cells, providing a promising new strategy to combat drug resistance.
This study identifies PRMTs as pivotal therapeutic targets in paclitaxel-resistant TNBC, and uncovers novel mechanisms by which PRMT inhibition induces cancer cell death through RNA splicing alterations and chromosomal instability. It offers a promising avenue for developing effective treatments to overcome paclitaxel resistance and improve the survival of TNBC patients.
More information: KeJing Zhang et al, A chemical screen identifies PRMT5 as a therapeutic vulnerability for paclitaxel-resistant triple-negative breast cancer, Cell Chemical Biology (2024). DOI: 10.1016/j.chembiol.2024.08.003