October 5, 2012 feature
Of enzymes and aging: Tryptophan metabolism plays key role in aging and age-related neurological diseases
(Medical Xpress)—In the battle against aging and age-related neurological diseases such as Parkinson's and Alzheimer's, a key factor has long appeared to be the toxicity of proteins which tend to aggregate. Recently, scientists at University of Groningen, The Netherlands identified the protein-coding gene TDO2 that encodes for tryptophan 2,3-dioxygenase – the enzyme that degrades tryptophan and thereby reduces its levels – as a metabolic regulator of age-related protein toxicity and lifespan in the eukaryotic nematode Caenorhabditis elegans. The researchers also showed that the regulation of lifespan occurred through evolutionarily conserved genetic pathways. The study demonstrated that TDO2 depletion increases tryptophan levels, while feeding C. elegans with extra L-tryptophan also suppresses toxicity. The researchers conclude that their findings suggest that TDO2 regulates proteotoxicity through tryptophan.
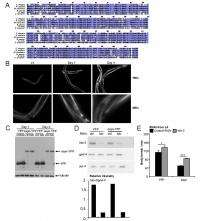
Dr. Ellen A. A. Nollen, doctoral student Annemieke T. van der Goot and 14 other researchers had to address a range of challenges in their study, starting with identifying TDO2 as a metabolic regulator of age-related α-synuclein toxicity in a C. elegans model. "One of the hallmarks of many neurodegenerative diseases," van der Goot tells Medical Xpress, "is the accumulation of misfolded proteins in inclusions in the brain." In the case of Parkinson's disease, she illustrates, the major component of these inclusions is alpha-synuclein, and the team had previously developed a C. elegans model expressing human alpha-synuclein fused to Yellow Fluorescent Protein (YFP) in their body wall muscle cells.
"We used this model to identify genetic factors involved in the formation of inclusions and found 80 genes that when knocked down increased the number of inclusions," van der Goot continues. "However, it's unclear whether these inclusions are toxic – they might even be protective. We therefore decided to test how the previously identified modifiers affected toxicity of alpha-synuclein."
Since alpha-synuclein is expressed in the muscle cells of the nematodes, and this causes a strong decline in muscle function as the animal's age, the scientists used a motility assay (a test that measures movement of the worms) to score for effects on toxicity. "We observed that many of the modifiers did not affect toxicity at all, thirteen increased toxicity, and three decreased toxicity. As has been suggested by others as well, these data indicate that the number of visible inclusions does not relate to toxicity."
The team obtained the strongest toxicity suppression by knocking down TDO2, thereby reducing expression levels of tryptophan 2,3-dioxygenase – the first and rate-limiting step tryptophan degradation. Moreover, they observed that TDO2 knockdown prevented a decline in muscle function in control animals as well. "We then wondered whether TDO2 could also be a general regulator of protein homeostasis and suppress toxicity of other misfolding proteins." To test this, they used C. elegans models for Alzheimer's and polyglutamine disease, finding that TDO2 knockdown would indeed suppress toxicity associated with these heterologous disease proteins. "Excitingly," van der Goot notes, "toxicity suppression wasn't restricted to models expressing disease proteins in the muscle, but was also observed in a neuronal polyglutamine model."
Finally, van der Goot adds, they next tested whether depletion of TDO2 would also be able to suppress the misfolding of endogenous metastable proteins – that is, proteins carrying point mutations that can fold normally when grown at the permissive temperature but misfold when shifted to a restrictive temperature. "We saw that TDO2 knockdown could suppress the toxicity of these endogenous metastable proteins as well and, importantly, TDO2 knockdown rescued a developmental defect in one of the temperature-sensitive mutants, showing that the protective effect of TDO2 depletion is not specific to motility."
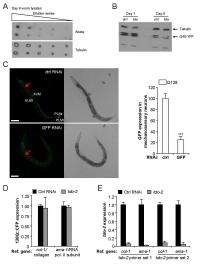
Addressing these considerable challenges involved a number of key insights. "In this study," van der Goot explains, "we show that TDO2 functions as a regulator of protein homeostasis and lifespan. Although correlative studies had previously suggested a possible link between tryptophan metabolites and aging-related diseases, recent studies in multiple model organisms – including C. elegans, fruit flies, and mice – have now firmly established tryptophan metabolism as a potent regulator of age-related pathologies. While much attention has focused on the neuroactive properties of metabolites that are formed during the degradation of tryptophan, we found that tryptophan levels itself might play an important role in modulating aging and protein homeostasis." Indeed, one of the study's strongest points is the use of genetic mutants for multiple enzymes in the kynurenine pathway downstream of TDO2 combined with the measurement of all downstream kynurenines metabolites – a systematic and comprehensive analysis had not previously been done.
In addition, the study suggests that TDO2 functions as a general regulator of protein homeostasis, implying that TDO2 acts as a metabolic switch of age-related protein homeostasis and lifespan. "Tryptophan is an essential amino acid that has to be taken up by food," says van der Goot. "Therefore, this amino acid is a good candidate to signal environmental conditions to pathways that adjust metabolism and regulate health of an animal. TDO2 is the main enzyme responsible for regulating levels of tryptophan, so turning this enzyme on and off can make it serve as a switch by regulating tryptophan levels."
Van der Goot also describes the team's planned next steps in their research. "Future studies will have to show whether our findings can be translated to other organisms. Moreover, we'd like to find out how TDO2 regulates protein homeostasis and longevity – in other words, what the downstream targets are."
The study's findings may also benefit other areas of research. "Regulation of TDO2 levels has been shown to play a role in cancer as well," van der Goot concludes. "We can therefore envision a benefit for food scientists or pharmaceutical scientists who could explore whether addition of a TDO2 inhibitor in the diet can promote a long and healthy life in mice."
More information: Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation, PNAS September 11, 2012, vol. 109 no. 37 14912-14917, doi:10.1073/pnas.1203083109
Copyright 2012 Medical Xpress
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.