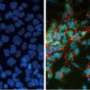
Scientists turns liver cells directly into neurons with new technique
(Medical Xpress) -- Fully mature liver cells from laboratory mice have been transformed directly into functional neurons by researchers at the Stanford University School of Medicine. The switch was accomplished with the introduction of just three genes and did not require the cells to first enter a pluripotent state. It is the first time that cells have been shown to leapfrog from one fundamentally different tissue type to another.
The accomplishment extends previous research by the same group, which showed in 2009 that it is possible to directly transform mouse fibroblasts, or skin cells, into neurons.
“These liver cells unambiguously cross tissue-type boundaries to become fully functional neural cells,” said Marius Wernig, MD, PhD assistant professor of pathology and a member of Stanford’s Institute for Stem Cell Biology and Regenerative Medicine. “Even more surprising, these cells also simultaneously silence their liver-gene expression profile. They are not hybrids; they are completely switching their identities.”
The cells make the change without first becoming a pluripotent type of stem cell — a step long thought to be required for cells to acquire new identities.
Wernig is the senior author of the research, published online Sept. 29 in Cell Stem Cell. Postdoctoral scholar Samuele Marro, PhD, is the first author of the study.
The researchers used a technique developed by Stanford bioengineer Stephen Quake, PhD, to analyze the gene expression profiles of individual hepatocytes (liver cells) and fibroblasts to show that both types of transformed cells not only begin looking and acting like true neurons, they also decisively shut down nearly all gene expression associated with their former, very different identities.
“This is fascinating,” said Wernig. “We can imagine ways that the three introduced factors could stimulate neural gene expression, but how do they also down-regulate two completely unrelated donor networks — those of skin and liver cells?”
Understanding how this down-regulation works will help scientists and clinicians determine whether these so-called transdifferentiated cells can be used to learn more about diseases or even be safely used in human therapy. It would not be good, for example, if newly derived neurons began to again express skin or liver proteins. It also may help researchers understand the process of development, during which cells commit to certain fates while also turning off other potential pathways.
Wernig and Marro began investigating whether hepatocytes could transform into neurons because the fibroblasts they first transformed into neurons in 2010 are a notoriously messy groups of cells. Fibroblasts can be found in almost any organ in the body and contain mixtures of cell types. This made it extremely difficult to identify a cell-of-origin for the resulting neurons and to figure out exactly how big of a developmental leap the cells were making.
In contrast, hepatocytes are fairly homogenous and well-defined. Developmentally speaking, they are also worlds away from neurons: Hepatocytes arise from one of three classes of embryonic tissue called the endoderm; neurons from the ectoderm. The remaining tissue, the mesoderm, is, for the most part, sandwiched between the two. To put it simply: Your innards mostly arise from endoderm, your nervous system and the outer layer of your skin from ectoderm, and your connective tissue and muscles from mesoderm. Transforming endodermal cells into ectodermal cells is a testament to the power of the transdifferentiation technique.
To accomplish the transformation of the hepatocytes, the researchers used a virus to introduce the same three genes that they used for the fibroblasts: Brn2, Ascl1 and Myt1l. As with the fibroblasts, the hepatocytes began to exhibit neuronal characteristics within two weeks, and express neuronal genes within three weeks. Simultaneously, the cells began to suppress the expression of liver-specific genes.
Marro and Wernig used a sophisticated cell-labeling technique to confirm that the new neurons had indeed arisen from the former liver cells, and Fluidigm dynamic polyermerase chain reaction assays to analyze gene expression patterns of individual neuronal cells. They found that even “true” neurons express low levels of liver genes in the form of transcriptional noise. However, the newly differentiated neurons did express marginally higher levels of the same genes.
“Although the donor gene program is dramatically shut down, there are some remnants of their former life, like a kind of a memory,” said Wernig. “But the vast majority of expressed genes demonstrate a clear dominance of the neuronal transcription program.” Furthermore, the fact that the newly derived neurons generate electrical signals and form junctions with other neurons, and that they exhibit no residual liver function, indicates that this memory has no functional relevance, according to Wernig.