Phase I trial begins using gene therapy and bone marrow stem cells in the treatment of brain cancer
University Hospitals (UH) Case Medical Center, Case Western Reserve University School of Medicine and Lentigen Corporation announced today the initiation of a novel Phase I clinical trial of LG631 gene therapy for the protection of hematopoietic stem cells (HSCs) from the dose limiting toxicity of chemotherapy with Temodar.
Approximately 17,000 Americans are diagnosed with glioblastoma every year and only two percent of them survive longer than five years — even with aggressive treatment. Glioblastoma (GBM) treatment generally begins with a surgical resection, followed by radiation therapy and then chemotherapy to destroy any remaining cancer cells. Temodar (temozolomide, Merck and Co., Inc.) is a standard treatment of glioblastoma, but dose-limiting bone marrow toxicity often accompanies such therapy.
In this first-of-its-kind study, researchers are investigating if LG631 can potentially improve tolerance and effectiveness of chemotherapy for GBM by preventing damage to bone marrow. The study will evaluate the safety of this treatment and its potential to enhance current GBM treatments.
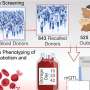
For this study, HSCs will be isolated from participating patients, transduced with LG631, an altered human-Methylguanine Methyltransferase (MGMT) gene to make them resistant to Temodar, and then infused back into the patient. The MGMT gene has been shown to repair damaged DNA. The specially designed Lentiviral vector (LG631) will be used to deliver the gene specifically to bone marrow stem cells that are susceptible to damage by drugs commonly used to treat cancer, thereby enabling patients to receive higher doses of Temodar with less severe side effects.
"Glioblastoma is a devastating disease and many patients do not benefit from standard therapy," notes Dr. Andrew Sloan, Director of the Brain Tumor and Neuro-Oncology Center, UH Case Medical Center and Associate Professor of Neurological Surgery and Pathology at Case Western Reserve University School of Medicine, who is leading the clinical trial. "This trial has the potential to change the way we treat GBM—particularly in patients whose bone marrow is sensitive or those whose tumors are likely to be resistant to standard therapies."
The LG631 vector was designed by Lentigen Corporation, a biotechnology company specializing in the development and manufacture of Lentiviral gene delivery technologies, and has been evaluated in animal models in collaboration with Stanton Gerson, MD, Director of the Case Comprehensive Cancer Center at Case Western Reserve University and Seidman Cancer Center at UH Case Medical Center. The application of the mutated MGMT gene for use in stem cell protection was discovered in Dr. Gerson's laboratory.
"The combination of the vector technology with the stem cell gene therapy approach is highly innovative," says Dr. Gerson. "This is the first time the combination will be used in cancer patients right after surgery. We are excited about this promising approach to enhance current treatments for glioblastoma."
"The initiation of this NCI-supported study represents an important milestone in the development of Lentigen's pipeline," said Tim Ravenscroft, CEO of Lentigen Corporation. "It is the first of several products which we expect to enter the clinic in the next 12 months. For patients with glioblastoma treated with Temodar, it is our hope that LG631 gene therapy of bone marrow stem cells can substantially improve patient outcomes in this devastating disease."
The project described was supported by Grant Number R42CA128269 from the National Cancer Institute and a grant to the Center for Stem Cell & Regenerative Medicine (CSCRM) from Ohio's Third Frontier Commission under its Research Commercialization Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.