Bacteria subverts immune response to aid infection
Listeria, one of the most deadly causes of bacterial food poisoning, subverts a normally protective immune response to spread its infection more effectively, according to new research at National Jewish Health. Immunologists Laurel Lenz, PhD, Peter Henson, PhD, and their colleagues report online April 26, 2012, in the journal Immunity that production of nitric oxide (NO) by activated macrophages, which is normally thought of as an infection-fighting response, actually helps Listeria monocytogenes to more efficiently disseminate between infected and neighboring uninfected cells.
"In the course of evolution, pathogens and their hosts engage in an ongoing arms race, responding to and countering each other's tactics to gain the upper hand," said Dr. Lenz. "In this case, Listeria has learned to evade a response that is normally protective and to do so in a way that substantially increases the spread of infection. Several other pathogens, including Rickettsia, Burkholderia, Vaccinia and HIV, spread throughout the host in a similar manner and may use similar strategies."
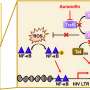
When Listeria or other pathogens first enter the body, receptors on white blood cells recognize general features of the pathogen and sound an early alarm that activates the innate immune response. When activated, macrophages and other innate immune cells can more readily prevent free-floating pathogens from surviving upon entering cells. However, these activated cells also release of nitric oxide (NO), an important signaling molecule that triggers several defense mechanisms.
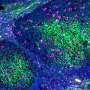
Dr. Lenz and his colleagues found that production of NO by activated cells helped to increase Listeria spread directly from cell to cell and replicate in its host. When Listeria spreads directly from cell to cell, it produces small buds on the surface of an infected cell. Neighboring cells that touch the infected cell absorb the buds containing the Listeria. Thus, the bacteria are transferred without ever entering the extracellular environment. The absorbed Listeria are initially contained within small bubbles, known as a vacuoles or phagosomes. Normally when a white blood cell absorbs a particle or organism, these phagosomes are targeted by a sort of cellular Death Star that fuses with them and destroys their contents. NO, however, delays the attack of these Death Stars, or lysosomes. This delay gives Listeria more time to escape the phagosome into the cell interior before it can be destroyed by the lysosomes.
"Delaying lysosome fusion with phagosomes tips the scale in favor of Listeria, allowing this pathogen to more effectively infect cells through cell-to-cell spread and thus to multiply in its host," said Dr. Lenz.