Liver fat gets a wake-up call that maintains blood sugar levels
A Penn research team, led by Mitchell Lazar, MD, PhD, director of the Institute for Diabetes, Obesity, and Metabolism at the Perelman School of Medicine, University of Pennsylvania, reports in Nature Medicine that mice in which an enzyme called histone deacetylase 3 (HDAC3) was deleted had massively fatty livers, but lower blood sugar, and were thus protected from glucose intolerance and insulin resistance, the hallmark of diabetes.
Insulin resistance occurs when the body does a poor job of lowering blood sugars. Typically, patients with obesity and type 2 diabetes have fatty livers, and the dogma in the field, says Lazar, is that the fatty livers contribute to the insulin resistance and diabetes in a vicious cycle. These findings are "a clear counterexample to this thinking," he says.
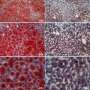
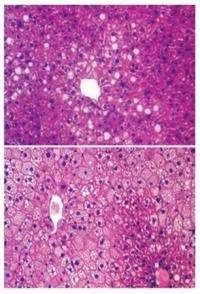
The researchers observed that the extra fat in the liver did not cause insulin resistance because it was sequestered in tiny lipid droplets inside individual liver cells, coated by a specific protein. The metabolites that would otherwise be used by the body to make glucose were re-routed to make fat, leading to reduced glucose in the bloodstream. The advantage of the lower blood sugar is tempered by the excess liver fat, which can lead to problems of its own, including liver failure.
Cells of high-fat-diet-induced fatty livers in wild-type mice were characterized by larger lipid droplets, but liver-specific HDAC3 knockout mice on a high-fat diet were characterized by smaller lipid droplets, even though the total lipid content increased versus the wild-type mice.
Why would the body have this re-routing process in the first place? The team looked to the circadian rhythm of the nocturnal mice for answers. When inactive during the day, mouse HDAC3 migrates to genes to turn off fat synthesis. This allows metabolites to make glucose for fueling the sleeping body. When waking, during the night, the mouse body makes a metabolic switch, anticipating the intake of food, and turns on fat synthesis for energy storage. The on-and-off cycle of HDAC3 is directly regulated by the internal circadian clock, and the system falls apart when HDAC3 is deleted.
The findings suggest that the cordoning off of lipids of the liver in many, tiny coated droplets helps to manage insulin resistance in the body. And, the findings cement the fact that HDAC3 is pivotal in integrating signals from the internal body clock to coordinate metabolism, especially in the liver, notes the first author Zheng Sun, PhD, postdoctoral fellow in the Lazar lab.
The findings demonstrate that fat itself is not necessarily all bad. "It matters a lot how fat is handled and stored," notes Lazar. "It also highlights the importance of complying with our internal circadian clock. For example, since our body does not anticipate food at night and is preparing to generate more glucose, night-time eating is likely to shoot up blood sugar and thus may contribute to diabetes."