Cervical cancer vaccine shows promise
A vaccine against cervical cancer, being developed by Inovio Pharmaceuticals Inc. of Blue Bell, Pa., produced positive results in a small sample of 18 women.
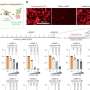
The vaccine prompted their bodies to produce T cells - a type of white blood cells - that, in a separate lab test, recognized cells with tumor proteins, and killed them.
The researchers, including a team from the University of Pennsylvania, say the paper in the journal Science is the first to show that a DNA vaccine alone produced a high level of immunity in people. At the same time, the researchers acknowledged that a working vaccine faces more trials and remains years away from an actual product.
Cervical cancer is the second most common type - after breast cancer - in women worldwide. Every year, about 470,000 women are diagnosed with cervical cancer. About half of them, mostly in developing countries, eventually die.
Unlike other cancers, it is caused by infection - in this case, some types of human papillomavirus, which also causes genital warts.
Vaccines to prevent HPV infection have been developed - Gardasil, by Merck & Co., and Cervarix, by GlaxoSmithKine.
"The problem is, the vaccines don't protect or help women who are already infected with the virus," said J. Joseph Kim, CEO of Inovio, which funded the study. He and several Inovio scientists participated in it.
In the United States, only about three in 10 teenage girls - the target group - are fully vaccinated, and many fewer in developing countries. Plus, many women were infected before the two vaccines were developed. Researchers have estimated that between 28 million and 40 million women worldwide have pre-cancerous HPV infections.
In the initial trial for the Inovio vaccine, called VGX-3100, 18 women who had been treated previously for lesions were injected with a vaccine made of DNA carrying a genetic code targeted to prompt the body to make a specific kind of T cell.
Those T cells were then removed and combined in a test tube with other cells from the women that displayed the tumor protein. In the lab setting, the T cells attacked and killed the other cells.
The results were exciting for David B. Weiner, a Penn professor of pathology and laboratory medicine, who also participated in the study.
"The T cells' ability to recognize and kill those targets in the lab suggests that they would now be able to do this against the women's own cancer cells in their bodies," he said.
Weiner's lab pioneered the use of DNA vaccines, a research field that looked promising 20 years ago, partly because things that may have side effects can be eliminated. They are nonlive, so there's no risk of them causing the disease. They don't replicate in the body.
But in the late 1990s, interest in the new vaccines flagged. In tests on larger animals and humans, the immune response was disappointing.
Now, said Weiner, "basically, this is the first paper that we're aware of that demonstrates that a DNA vaccine on its own in humans could produce this quality or magnitude of immunity."
"It opens up a lot of exciting avenues of study," he added. In the paper, Weiner reported compensation from Inovio and other drug companies.
One of the differences this time is that the vaccine was delivered along with a small electric pulse, believed to greatly increase the efficiency of the vaccine.
Stanley A. Plotkin, an emeritus professor at Penn whose career has been in vaccine development, said the study is important because vaccines have traditionally been prophylactic, preventing the disease before it occurs.
"In this case," he said, "we're talking about therapeutic vaccines, used in people who already have a disease in order to eliminate it or to control it."
Also, he said, the current research goes a long way toward "making a DNA vaccine practical and useful in humans, rather than only in animal models."
Plotkin, a co-discoverer of the rotovirus vaccine, is a consultant to vaccine manufacturers, including Inovio, but was not involved in the study.
Most side effects of the vaccine were minimal and deemed unrelated to the treatment, the Science paper reported.
After nine months - the official conclusion of the study - the participants were still producing T cells, Kim said. However, he said, the effect seems to be more durable than that. Tests up to three years later showed the vaccination was still working.
Inovio is currently conducting phase II trials on about 150 women worldwide who have untreated precancerous lesions. Kim said the results of that study are expected by the end of 2013.
Kim said it could be four to six years before the vaccine, if it ultimately proves effective, could be commercially available.
(c)2012 The Philadelphia Inquirer
Distributed by MCT Information Services