Many causes for learning lags in tumor disorder
(Medical Xpress)—The causes of learning problems associated with an inherited brain tumor disorder are much more complex than scientists had anticipated, researchers at Washington University School of Medicine in St. Louis report.
The disorder, neurofibromatosis 1 (NF1), is among the most common inherited pediatric brain cancer syndromes. Children born with NF1 can develop low-grade brain tumors, but their most common problems are learning and attention difficulties.
"While one of our top priorities is halting tumor growth, it's also important to ensure that these children don't have the added challenges of living with learning and behavioral problems," says senior author David H. Gutmann, MD, PhD, the Donald O. Schnuck Family Professor of Neurology. "Our results suggest that learning problems in these patients can be caused by more than one factor. Successful treatment depends on identifying the biological reasons underlying the problems seen in individual patients with NF1."
The study appears online in Annals of Neurology.
According to Gutmann, who is director of the Washington University Neurofibromatosis Center, scientists are divided when considering the basis for NF1-associated learning abnormalities and attention deficits.
Mutations in the Nf1 gene can disrupt normal regulation of an important protein called RAS in the hippocampus, a brain region critical for learning. Initial work from other investigators had shown that increased RAS activity due to defective Nf1 gene function impairs memory and attention in some Nf1 mouse models.
However, earlier studies by Gutmann and collaborator David F. Wozniak, PhD, research professor in psychiatry, showed that a mutation in the Nf1 gene lowers levels of dopamine, a neurotransmitter involved in attention. In this Nf1 mouse model, Gutmann and his colleagues found that the branches of dopamine-producing nerve cells were unusually short, limiting their ability to make and distribute dopamine and leading to reduced attention in those mice.
The new research suggests that both sides may be right.
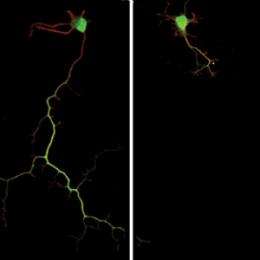
In the latest study, postdoctoral fellow Kelly Diggs-Andrews, PhD, found that the branches of dopamine-producing nerve cells that normally extend into the hippocampus are shorter in Nf1 mice. As a result, dopamine levels are lower in that part of the brain.
Charles F. Zorumski, MD, the Samuel B. Guze Professor and head of the Department of Psychiatry, showed that the low dopamine levels disrupts the ability of nerve cells in the hippocampus to modulate the way they communicate with each other. These communication adjustments are a primary way the brain creates memories.
Researchers then found that giving Nf1 mice L-DOPA, which increases dopamine levels, restored their nerve cell branch lengths to normal and corrected the hippocampal communication defect. L-DOPA also eliminated the memory and learning deficits in these mice.
"These results and the earlier findings suggest that there are a variety of ways that NF1 may cause cognitive dysfunction in people," Gutmann says. "Some may have problems caused only by increased RAS function, others may be having problems attributable to reduced dopamine, and a third group may be having difficulties caused by both RAS and dopamine abnormalities."
To customize patient therapy, Gutmann and his colleagues are now working to develop ways to quantify the contributions of dopamine and RAS to NF1-related learning disorders.
More information: Diggs-Andrews KA, Tokuda K, Izumi Y, Zorumski CF, Wozniak DF, Gutmann DH. Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice. Ann Neurol. 2012 Oct 30.