Ruling the airways: Notch controls bronchial cell fates and distributions
Nestled deep within the body, the epithelial lining of the respiratory system is nonetheless seriously exposed. Its direct contact with environmental air necessitates protective mechanisms that both seal off the respiratory tract from other compartments of the body and neutralize microbial invaders. This is achieved by the coordinated action of the functionally specialized various cell types that make up the lining of the airway. These respiratory cell populations include major ciliated cells, exocrine Clara cells, and neuroendocrine (NE) cells, all of which are generated by a common epithelial progenitor cell type during embryogenesis.
One interesting feature of lung histology is that the relative distributions of these cell types varies along the proximal-distal axis, with ciliated cells capable of sweeping away microscopic particles and organisms more common near the mouth, and secretory Clara cells that help maintain humidity and bronchiole structure increasing in deeper regions of the lung. Neuroendocrine cells are less common than ciliated and Clara cells, and are found in clusters throughout the lung epithelium. Despite their importance, the mechanisms by which these spatial changes in lung cell distribution are regulated remain largely a mystery.
Mitsuru Morimoto, Team Leader of the Laboratory for Lung Development, working with colleagues at the National Institute of Genetics and Washington University of St. Louis, has now shown that Notch signaling controls the differentiation and distribution of these three lung epithelial cell types in mouse. Published in Development, this work sheds new light on how the diversity of lung cells is established and maintained.
The Notch signaling pathway is triggered by interactions between ligands and receptors bound within cell membranes, making it a much more local affair than signaling involving secreted molecules that diffuse away from cells. Morimoto had previously noted that when Notch signaling is reduced during development, the number of Clara cells in the lung epithelium decreases, while ciliated cells expand in number.
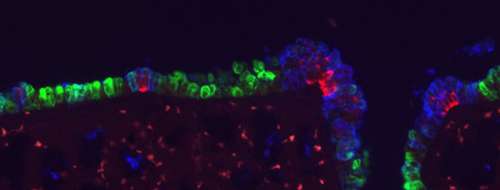
Other work had also shown that the Notch pathway ligand Jag1 is expressed in ciliated cells, which it was suggested might induce Clara cell differentiation in neighboring cells. To work out the details of how Notch works in lung cell fate determination, Morimoto in this most recent study tested how ablating the Notch receptors Notch1 – 3 affected the relative distributions of epithelial cell types in the airway. His first finding came as something of a surprise – Clara cell differentiation relies on Notch2, not Notch1 as previously believed.
Morimoto further observed that simultaneous deletion of all three receptors caused the size of neuroendocrine cell clusters to increase, an effect not seen when these factors were knocked out individually or in other combinations, suggesting that Notch1 – 3 have additive functions in the regulation of NE cell differentiation. Even more intriguingly, Notch activity was not observed in the neuroendocrine cells themselves, but rather in a distinct population of their neighbors, apparently representing a hitherto unidentified cell type, which the team dubbed SPNC cells, after a characteristic gene expression profile (SSEA-1+ peri-pNEB N1ICD+ CC10-). Morimoto found that these SPNC cells are maintained by juxtacrine Notch signaling from adjacent NE cells, suggesting that once neuroendocrine cells have been induced, they function to maintain local SPNC cells, which in turn provide signals that regulate the size of NE cell clusters.
"The combinatorial effects of Notch factors in the airway epithelium may be the mechanism underlying the distribution of ciliated, Clara and neuroepithelial cells. While the ciliated/Clara selection is sensitive to dosage of Notch2, NE cells are regulated synergistically by the combination of Notch1 – 3, which may account for various pattern of ciliated/Clara cells and the uniform distribution of NE cell cluster throughout the epithlieum," says Morimoto. "In future studies, we will be interested in looking more deeply into how the Notch signaling works in Clara cell differentiation, and inducers of NE cells as well."
More information: dev.biologists.org/content/139/23/4365.long
















