July 15, 2013 feature
All together now: Novel mechanism directs both dendritic and axonal growth in the same neuron
(Medical Xpress)—Although the brain-as-computer metaphor is flawed in several ways, dendrites and axons may be considered respectively as a neuron's input and output compartments – and determining how they differentiate during neuronal development is critical in understanding neural circuit assembly as well as in correcting and preventing defective and damaged neurons. Recently, scientists at the University of Michigan demonstrated that a single molecular pathway in Drosophila (fruit flies) controls both dendritic and axonal growth, doing so by focusing on dual leucine zipper kinase (DLK) – a key molecule in this pathway. While DLK is a key regulator of axon growth and regeneration, the new study demonstrates for the first time its role in dendritic growth. The researchers conclude that their findings may lead to a method for promoting axon regeneration without affecting dendritic growth, and suggest that their results provide a new perspective for understanding neuronal compartmentalization and morphology.
Prof. Bing Ye
discussed the research he and his co-authors – Xin Wang, Jung Hwan Kim, Mouna Bazzi, Sara Robinson, and Catherine A. Collins – published in their paper. "We encountered two main challenges in our investigation of a novel
What makes the system even more complex, Ye points out, is that each neuron typically grows many dendritic and axonal branches, so that without single cell resolution, it is almost impossible to discern the shapes of dendrites and axons of individual neurons. "Secondly, the distances between the axonal terminals and the dendritic branches are usually long," Ye adds, "making it difficult for us to image all axonal and dendritic branches of a single neuron in vivo."
Another challenge the researchers encountered, says Ye, was that in order to study signaling pathways that control dendritic and axonal development in a neuron, it is essential to manipulate genes in the neuron of interest. Otherwise, signaling defects in the surrounding cells and tissues may affect dendrites or axons, thereby complicating the interpretation of results. "In other words, in addition to single cell resolution for labeling all dendritic and axonal branches of a neuron," Ye says, "we needed single cell resolution for genetic manipulations in order to study the molecular mechanisms underlying the differential development of dendrites and axons."
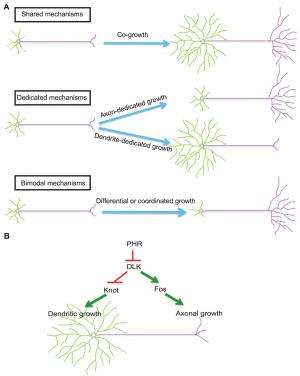
Relatedly, enhancing DLK activity promotes axonal growth, but reduces dendritic growth. "We found that DLK suppresses dendritic growth in part by inhibiting the transcription factor Knot. By maintaining Knot activity while activating DLK, we were able to promote axon regeneration without affecting dendritic growth."
Ye describes the three key techniques that were crucial for their in vivo study of the signaling mechanism separating dendritic growth from axonal growth in the same neurons:
· Genetic mosaic techniques, including mosaic analysis with a repressible cell marker (MARCM) invented by Liqun Luo and his then-postdoctoral student Tzumin Lee at Stanford1, provided single cell resolution for not only labeling of neuron morphology but also genetic manipulations.
· During postdoctoral research in the laboratory of Yuh Nung Jan at UCSF, Wesley Grueber (now at Columbia) and Ye generated a highly-specific marker that allows labeling and genetic manipulations specifically in one type of neurons2. Using this marker in their previous and current studies, Ye and his colleagues were able to carry out genetic screens to search for mutants with defects that were different between dendrites and axons.
· The scientists chose Drosophila to carry out their study in addition to the powerful genetic analysis that Drosophila offers, its nervous system is much smaller than vertebrate model systems – making it easier to track all dendritic and axonal branches of a single neuron.
The researcher's findings not only reveal a previously unknown function of the conserved DLK pathway in controlling dendrite development, but also provide a novel paradigm for understanding how neuronal compartmentalization and the diversity of neuronal morphology are achieved. "Ever since Ramon y Cajal described the shapes of neurons in both vertebrates and invertebrates3, the tremendous diversity of neuronal morphology has inspired generations of experimentalists and theorists to decipher the underlying mechanisms that create such diversity," Ye explains.
Some neurons, such as cerebellar Purkinje cells, grow a lot of dendrites but only a few axonal branches, whereas some other neurons – for example, cerebellar granule cells – grow a lot of axons but only a few short dendritic branches. These different shapes serve distinct neuronal functions. "We don't know how these different shapes are formed," Ye notes. "Our findings show that in Drosophila somatosensory neurons, the ratio between the size of dendrites and that of axons is determined by the activity of the dual leucine zipper kinase DLK. This provides an attractive model that explains how dendrite-axon ratios can be regulated." In addition, their results also present a model explaining how dendrites and axons, two subcellular compartments of the same cell, are differentially regulated – and that this is through two distinct transcriptional programs.
The scientists are now developing in vivo systems to study the differential development of dendrites and axons in mice, and are also developing imaging techniques to interrogate molecular signaling events at subcellular resolution. Specifically, based on their findings, they researchers hypothesize that the control of the dendrite-axon ratio by DLK might be a general mechanism. "This is an exciting possibility because if true it would be a unifying theory that explains at least one aspect of the tremendous diversity of neuronal morphology," Ye comments. "We're eager to test this hypothesis in both fruit flies and mice."
Beyond their own research, Ye notes that cell polarity or subcellular compartmentalization is a fundamental issue in biology. "Our study demonstrates a previously unknown mechanism that controls the development of different subcellular compartments in a cell."
More information: Bimodal Control of Dendritic and Axonal Growth by the Dual Leucine Zipper Kinase Pathway, PLoS Biology 11(6): e1001572 (2013), doi:10.1371/journal.pbio.1001572
Related
1Mosaic Analysis with a Repressible Neurotechnique Cell Marker for Studies of Gene Function in Neuronal Morphogenesis, Neuron Vol. 22, 451–461, March 1999 (PDF)
2Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion, Current Biology Volume 13, Issue 8, 618-626, 15 April 2003,
10.1016/S0960-9822(03)00207-0 (PDF)
3Histology of the Nervous System of Man and Vertebrates: Oxford University Press, USA (1995)
© 2013 Medical Xpress. All rights reserved.