With three first-in-human trials, therapeutic stem cell science takes a bold step at UC San Diego
A 26-year-old woman paralyzed after a motor vehicle accident a year ago has successfully undergone a first-in-human experimental procedure to test whether neural stem cells injected at the site of a spinal cord injury is safe and could be an effective treatment.
The procedure, conducted on Sept. 30 under the auspices of the Sanford Stem Cell Clinical Center at UC San Diego Health System and in collaboration with Neuralstem, Inc., a Maryland-based biotechnology firm, is the first of four in the Phase I clinical trial. Post safety testing, it's hoped that the transplanted neural stem cells will develop into new neurons that bridge the gap created by the injury, replace severed or lost nerve connections and restore at least some motor and sensory function.
The patient, whose identity remains confidential for privacy reasons, has been discharged and is recovering without complication or adverse effects at home, said Joseph Ciacci, MD, principal investigator and neurosurgeon at UC San Diego Health System.
The spinal cord injury trial is one of three recent ground-breaking stem cell efforts at UC San Diego, supported by the Sanford Stem Cell Clinical Center, to make the significant leap from laboratory to first-in-human clinical trials.
Last month, researchers at UC San Diego Moores Cancer Center and the Sanford Stem Cell Clinical Center launched a novel Phase I trial to assess the safety of a monoclonal antibody treatment that targets cancer stem cells in patients with chronic lymphocytic leukemia, the most common form of blood cancer.
And later this month, the first patient is scheduled to receive an unprecedented stem cell-based therapy designed to treat type 1diabetes in another Phase I clinical trial at UC San Diego.
"What we are seeing after years of work is the rubber hitting the road," said Lawrence Goldstein, PhD, director of the UC San Diego Stem Cell program and Sanford Stem Cell Clinical Center at UC San Diego Health System. "These are three very ambitious and innovative trials. Each followed a different development path; each addresses a very different disease or condition. It speaks to the maturation of stem cell science that we've gotten to the point of testing these very real medical applications in people."
To be sure, Goldstein said, the number of patients involved in these first trials is small. The initial focus is upon treatment with low doses to assess safety, but also with hope of patient benefit. As these trials progress – and additional trials are launched – Goldstein predicts greater numbers of patients will be enrolled at UC San Diego and the Sanford Stem Cell Clinical Center and elsewhere.
"Clinical trials are the safest way to pursue potential therapies. You want to prove that a new therapy will work for more than just a single, random patient."
While stem cell-based trials are beginning to emerge around the country, Goldstein noted that San Diego continues to assert itself as a stem cell research hub and a leading force for translating basic discoveries into medical applications, now and in the future.
"These innovative trials are the result of some truly rare features you find at UC San Diego and in the region," he said. "There is a unique sense of collaboration and communication here among scientists in academia, clinical medicine and the biotechnology industry. An enterprise like the Sanford Center can promote and accelerate the very complex processes of research, development and testing so that the right people make the right connections and the right ideas and trials get fast-tracked, but in a way that ensures fundamentally the safety of patients while striving for benefit."
More about the three trials:
Neural Stem Cell Transplants and Spinal Cord Injuries
The Neuralstem Phase I clinical trial, conducted over five years with four patients, is designed to assess the safety and efficacy of an approach that might, it is hoped, someday be a treatment for paralyzing spinal cord injuries.
In pre-clinical studies, Ciacci and Martin Marsala, MD, a professor in the Department of Anesthesiology at UC San Diego School of Medicine and the Sanford Consortium for Regenerative Medicine, and colleagues grafted human neural stem cells into rats with spinal cord injuries. The introduced cells showed extensive growth and connected to remaining nerve cells near the injury site, resulting in significantly improved motor function with minimal side effects in animal models.
The goal now is to determine whether similar effects occur in human patients. The researchers will also test for possible therapeutic benefits, such as reduced paralysis and improvements in motor and sensory function, bowel and bladder function and pain levels.
VC-01 and Type 1 Diabetes
In collaboration with ViaCyte, Inc., a San Diego-based biotechnology firm specializing in regenerative medicine, UC San Diego researchers led by principal investigator Robert Henry, MD, professor of medicine in the Division of Endocrinology and Metabolism at UC San Diego and chief of the Section of Endocrinology, Metabolism & Diabetes at the Veterans Affairs San Diego Healthcare System, have launched the first-ever Phase I-II clinical trial of a stem cell-derived therapy for patients with Type 1 diabetes. The first procedure is planned for later this month, with a second tentatively scheduled in mid-November.
Type 1 diabetes mellitus is a life-threatening chronic condition in which the pancreas produces little or no insulin, a hormone needed to allow glucose to enter cells to produce energy. It is typically diagnosed during childhood or adolescence, but can also strike adults. Though far less common than Type 2 diabetes, which occurs when the body becomes resistant to insulin, Type 1 may affect up to 3 million Americans with emotionally and financially devastating consequences. Standard treatment involves daily injections of insulin and rigorous management of diet and lifestyle. Currently, there is no cure.
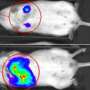
The 2-year trial will involve approximately 40 study participants at four to six testing sites, with San Diego being first. The trial will assess the safety, tolerability and efficacy of varying doses of VC-01, which involves implanting specially encapsulated embryonic stem cell-derived cells under the skin of patients where it's hoped they will safely mature into pancreatic beta and other cells able to produce a continuous supply of needed insulin and other substances.
Development and testing of VC-01 is funded, in part, by the California Institute for Regenerative Medicine (CIRM), Sanford Stem Cell Clinical Center and JDRF, formerly known as the Juvenile Diabetes Research Foundation. Clinical testing and coordination is provided by UC San Diego Clinical and Translational Research Institute.
Cirmtuzumab and Leukemia
Researchers at UC San Diego Moores Cancer Center and the Sanford Stem Cell Clinical Center have launched a Phase I human clinical trial to assess the safety and efficacy of a new monoclonal antibody for patients with chronic lymphocytic leukemia (CLL), the most common form of blood cancer in adults.
The drug, called cirmtuzumab, targets a molecule called ROR1 that normally is used only by embryonic cells during early development, but which is abnormally exploited by cancer cells to promote tumor growth and spread, otherwise known as metastasis. Metastasis is responsible for 90 percent of all cancer-related deaths.
Because ROR1 is not used by normal adult cells, scientists believe it is a unique marker of cancer cells in general and cancer stem cells in particular. ROR1 appears to drive tumor growth and disease spread and scientists think that presents an excellent novel target for anti-cancer therapy.
Cirmtuzumab was developed at Moores Cancer Center in the laboratory of Thomas Kipps, MD, PhD, who led this effort as one of six projects initially funded through CIRM's HALT leukemia grant to co-principal investigators Dennis Carson, MD, and Catriona Jamieson, MD, PhD. The drug's name acknowledges CIRM's continued support in a "Disease Team III" award, which provides some of the resources needed for a clinical trial. The Leukemia and Lymphoma Society has also provided additional support.
The trial will involve patients with relapsed or refractory CLL receiving an intravenous infusion every 14 days at Moores Cancer Center, followed by regular monitoring and clinic visits to assess efficacy and identify and manage any adverse effects. Initial treatment is planned for two months.