Project aims to help brain fix itself

A Rice University project to decipher how neurons form networks aims to help injured brains heal themselves.
Researchers at Rice funded by the National Science Foundation are combining experiments and computational analysis to learn how the brain organizes itself. Ultimately, they want to know if they can direct the growth of new neurons to treat stroke and neurodegenerative diseases.
The effort involves the combined talents of Rice experts in systems biology, nanotechnology and developmental biology. They are led by bioengineer Amina Qutub, who studies protein signaling and hypoxia (oxygen-starvation) and their effects on the brain. Joining her are electrical engineer Jacob Robinson, whose unique microfluidic devices help characterize the electrical properties of living cells, and developmental biologist Daniel Wagner, who develops zebrafish models of human disease.
The federal government is funding their work as part of President Barack Obama's BRAIN Initiative, which recently announced $13.1 million in grants for fundamental research.
"For a long time, brain cells were thought of as static, that you were born with the same brain cells you die with," said Qutub, an assistant professor of bioengineering. "But a lot of new research shows we're able to regenerate nerve cells through stem-cell-like neural progenitors."
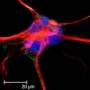
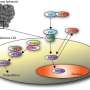
The researchers are studying how neural progenitor cells form neural networks through the coordination of chemical signals, spatial patterns and electrical activity. "While each one of these components has been studied separately, a big unanswered question is how these three modalities of cell communication are coupled," Qutub said.
"We're focusing on how neural progenitors become functional neural networks," she said. "That process is critical for regenerating nerve tissue. There's still debate over where the progenitors are located and how they're recruited to areas of injury, but we know they're present in the brain."
Once the researchers understand the mechanism, they hope to enhance it. "After a stroke, for instance, we want to be able to help regenerate local areas that were damaged and recover brain function," Qutub said. "At the moment, the area of damage around a stroke is often too large for self-repair."
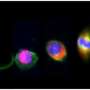
Qutub's team will first study human cells in the lab, mimicking how they behave in the brain as closely as possible. "We're breaking the process down like any engineer would, to reverse-engineer what happens as neural progenitors become neurons and connect as a network," she said. "What proteins are involved? What structural and electrical changes do they go through as they differentiate into fully functional neurons?"
By coupling new image-based and machine learning methods, the Qutub lab will identify unique cell phenotypes based on biochemical, electrical and structural communications that relate to the function of the neural networks they form. A dynamic computer model derived from this quantitative analysis will be used to address the second goal of the grant: to characterize how single-cell behavior relates to neural network properties.
To avoid measuring electrical changes in the cells through the established but far slower patch-clamp method, the researchers will test cells' electrical activity with a new high-throughput device being developed in Robinson's lab. The technique rapidly characterizes the electrical properties of live cells and then sorts them for further molecular and genetic analysis.
"As a neuron develops, its protein expression and morphology changes," said Robinson, an assistant professor of electrical and computer engineering. "We hope to connect these changes to electrical measurements that tell us if a neuron is mature enough to produce an action potential." Action potentials are the measurable activity of the voltage-gated ion channels cells use to communicate with each other.
He said his lab will record electrical activity in neural progenitor cells at different points in their development to see how they mature to a functional state. "Identifying the molecular pathways involved in the differentiation process is really important," he said. "If we can couple this to electrical activity, then we can come up with an accurate model of how the cell makes decisions during differentiation."
Wagner's zebrafish lab employs the transparent embryos to reveal biological processes in living animals. "Through Dan's work, we want to view processes in intact brains," Qutub said. "How do you look at this in a living organism? Well, you can't do that in humans, but in zebrafish, we can watch the neural progenitor cells become neurons."
One goal of the grant is to identify the role proteins like those found in hypoxic response pathways play in neural differentiation. Wagner is already developing zebrafish that will allow the researchers to track functional responses to a particular molecule, the HIF-2 alpha protein. "We see this molecule and its cofactors as core regulators of human neural progenitor cells and neuron function," Qutub said. "It's involved in hypoxic response, and we believe it's part of a signaling pathway responsible for some neurological disorders. We also believe it contributes to the process of turning neural progenitor cells into fully active neural networks."
"We plan to manipulate the activity of those molecules Amina identifies as potential key regulators and see what they do," said Wagner, an associate professor of biosciences. "We can look in vivo and say, 'If we change the activity of this pathway in this cell at this time, how does that affect the output of a particular pathway?'"
Qutub expects that she and her colleagues will share what they learn through events at Rice as well as an open-source image analysis toolkit for the neuroengineering community and an international, crowd-sourced neuronal network design challenge.