Research suggests new model for cancer metastasis
Scientists at UC San Francisco have been able to directly observe, for the first time, how invasive cancer cells create a beachhead as they migrate to the lung in a mouse model of metastatic cancer. What they saw was utterly surprising: early "pioneer" cancer cells that lodge in the lung generally die, but first they shed zombie-like particles that move around on their own and get gobbled up by waves of immune cells. Many of these immune cells, as if infected by the cancer particles, then burrow into the lung tissue, opening up space for future cancer cells floating through the blood to settle down safely and form new metastatic colonies.
"We had always thought that invasive cancer cells that got into a healthy tissue either lived and become metastatic or died and went down the drain," said UCSF's Matthew "Max" Krummel, PhD, professor of pathology and senior author of the new study, published in the March 16 online edition of Nature. "To our surprise we see that as cancer cells fragment and die, they're turning immune cells into partners and paving the way for the next cancer cell that comes along."
Krummel and his team believe this fundamentally new understanding of how early interactions between pioneer cancer cells and the immune system set the stage for metastatic cancer will lead to better approaches to treating and preventing invasive cancer in humans.
Microscopy breakthrough allows first video of arrival of invasive cancer in living lung
Invasive metastases in crucial organs like the lung, brain, and liver are the cause of the vast majority of cancer deaths, but until recently the process of metastasis itself has been poorly understood. Cancers are known to co-opt immune cells to soften up target tissues for metastatic invasion, but it hasn't been clear exactly how and why the body's protective cells turn traitor. One dominant theory has suggested that tumors produce molecular signals that reprogram the immune system and make it easier for new cancer colonies to take root, but how these signals actually interact with the immune response in target tissues is yet to be determined.
Few researchers have been able to directly observe the first stages of metastatic invasion to understand why some new cancer colonies flourish and others wither. But in recent years, researchers in Krummel's lab have perfected a system for stably imaging cancer cells in the lungs of mice during the first 24 hours after their arrival using 2-photon microscopy. This is a notable achievement, Krummel explains, because lungs move several millimeters back and forth with each breath, which would typically make it impossible for researchers to observe tiny cancer cells a thousandth of that size.
"Prior to beginning these studies it was as if the circulating tumor cells entered a black box and emerged as essentially mature metastases," said UCSF postdoctoral researcher Mark B. Headley, PhD, lead author of the new paper. "We now quite literally have a window into those earliest moments of metastatic development."
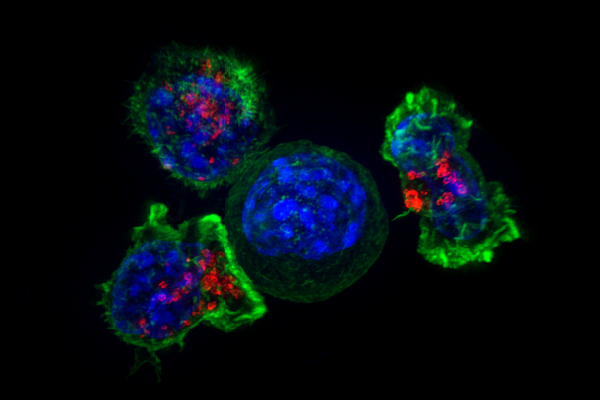
Headley and colleagues in Krummel's lab injected melanoma cells into bloodstream of mice and tracked the arrival of these cancer cells in the lungs, where they observed a bizarre and macabre scene unlike anything they had imagined. The early invaders themselves were blown to bits by the power of the blood flowing by, but their shredded remains took on a life of their own.
These fragments, which Krummel calls "headless horsemen," crawl along the capillary walls and fly through the blood deeper into the lung. The researchers also observed immune cells, alerted to the hubbub, mobbing the zombie cancer fragments and gobbling them up. But these cellular first responders soon began to act very strangely, leaving the capillaries and appearing to create protective nests for future cancer cells within the tissue of the lung itself.
"It's like the Old West," Krummel said. "Pioneers didn't always survive, but our civilization slowly progressed west by building on the foundation laid down by those early settlers. It's a totally new way of seeing metastasis, and just goes to show that by really looking at something properly for the first time, you can launch a thousand hypotheses that would never have occurred to you otherwise."
Insight into immune response suggests possibilities for treatment
Not all immune cells are taken in by the zombie cancer particles, Krummel and colleagues have found. After injecting cancer cells into mice, the researchers observed several distinct waves of immune cells arriving in the lung. Many early-responding immune cells – such as monocytes and macrophages – go on to help later cancer cells get established. Dendritic cells, on the other hand, typically arrive late to the scene, but appear to recognize the cancer as a threat: After ingesting cancer cell particles, they travel to the mouse's lymph nodes and activate other immune cells capable of returning to the lung and attacking any incipient metastatic colonies.
This realization spurred a new hypothesis about why some invasive cancers take hold and others don't, Krummel said. "In a successful metastasis, we think there's an imbalance, where too many cells are getting programmed by the tumor to accept cancer cells as harmless, and too few are getting the message that these cells are dangerous or different."
This possibility opens the door for new therapeutic approaches for patients at risk of developing metastatic cancer, he said, for instance by trying to suppress the gullible kinds of immune cells in order to prevent them from helping the cancer take root while at the same time enhancing the activity of the more astute dendritic cells to promote an appropriate immune response to get rid of any new colonies.
"By watching these events unfold we have revealed what we believe to be a critical line of communication between the tumor cell and its new home," Headley said. "We can now begin to study exactly what types of messages are packaged up in these particles and even more importantly how the immune response interprets those messages. Armed with that knowledge someday we will hopefully be able to co-opt or piggyback on those tumor-derived missives for the purposes of better therapies."
More information: Mark B. Headley et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung, Nature (2016). DOI: 10.1038/nature16985