Study discovers new HIV vaccine target
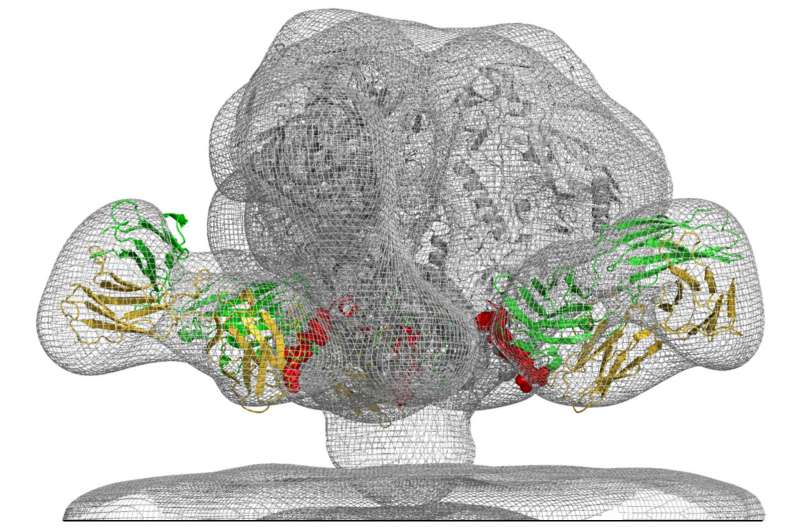
A team led by scientists at the National Institutes of Health (NIH) has reported a research trifecta. They discovered a new vulnerable site on HIV for a vaccine to target, a broadly neutralizing antibody that binds to that target site, and how the antibody stops the virus from infecting a cell. The study was led by scientists at the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases, part of NIH.
The new target is a part of HIV called the fusion peptide, a string of eight amino acids that helps the virus fuse with a cell to infect it. The fusion peptide has a much simpler structure than other sites on the virus that HIV vaccine scientists have studied.
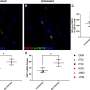
The scientists first examined the blood of an HIV-infected person to explore its ability to stop the virus from infecting cells. The blood was good at neutralizing HIV but did not target any of the vulnerable spots on the virus where broadly neutralizing HIV antibodies (bnAbs) were known to bind.
The researchers isolated a powerful bnAb in the blood that they named VRC34.01, and found that it binds to the fusion peptide and a sugar molecule. The scientists then crystallized the antibody while it was bound to the virus. This allowed them to characterize in atomic-level detail how VRC34.01 attaches to HIV and revealed that the antibody stops the virus from infecting a cell by binding to a key cell-surface molecule.
The scientists also report that it is not unusual for the immune system to try to stop HIV from infecting a cell by attacking the fusion peptide. When they screened the blood of 24 HIV-infected volunteers, they found that blood samples from 10 people targeted a similar binding site as VRC34.01.
The researchers are now working to create a vaccine designed to elicit antibodies similar to the VRC34.01 antibody.
The study is published in the journal Science.
More information: "Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody," DOI: 10.1126/science.aae0474