New research adds evidence on potential treatments targeting amyloid beta in Alzheimer's
New research findings from the Center for Cognitive Neurology at NYU Langone Medical Center could provide additional clues for future treatment targets to delay Alzheimer's disease and related dementias. This is according to the group's latest findings that will be presented at the Alzheimer's Association International Conference (AAIC), July 24 to July 28 in Toronto.
Alzheimer's disease, the most common form of dementia, is an incurable, degenerative disease that causes problems with memory, thinking and behavior. Alzheimer's and other dementias affect 47 million people worldwide and 5.3 million Americans, numbers that are expected to triple by 2050, according to the Alzheimer's Association. At this time, there is no cure—but research such as that presented at the AAIC meeting is aimed at finding ways to delay symptom onset and improve quality of life for patients, and may ultimately pave the way for more treatments.
New Target for Prevention
New research in mice by NYU Langone researchers may pave the way for clinical trials to test medications known as Carbonic Anhydrase Inhibitors (CAIs) as potential treatments for Alzheimer's, targeting the mechanism thought to be behind the neural and vascular death often associated with the disease.
It is now widely accepted that mitochondrial dysfunction—a destruction of the organelles that regulate energy metabolism and death in a cell - triggers the progression of the neuronal and vascular death seen in many Alzheimer's patients. Mitochondrial dysfunction is caused by a buildup of amyloid beta proteins, which, in turn leads to plaque accumulation in the brain.
In their study, NYU Langone researchers found that CAI medications—previously approved by the U.S. Food and Drug Administration (FDA) for other conditions like glaucoma—target the mechanism behind this dysfunction.
"Therapies aimed at preventing mitochondrial failure may represent promising new strategies as we search for a cure for this devastating disease," says lead study author Silvia Fossati, PhD, an assistant professor of neurology and psychiatry at NYU Langone.
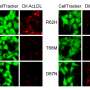
For the study, Fossati and colleagues looked at two FDA-approved CAIs: methazolamide and acetazolamide. For the first time, these medications were tested in cell cultures and mouse models that showed a buildup of amyloid-beta protein in the brain, known as amyloidosis. In the mice with amyloidosis, the researchers showed for the first time a positive effect of these drugs on memory, amyloid deposition, and activation of enzymes called caspases that drive cell death mechanisms in the brain.
The researchers believe that the protective effects of these compounds may be due to their prevention of mitochondrial dysfunction, as well as their known effects as activators of cerebral blood flow, which induce more efficient elimination of amyloid beta proteins from the brain. Further studies aim to test similar compounds in animal models, and since these drugs are already FDA-approved, Fossati adds that future research may involve the planning of a fast-track clinical trial in early stage Alzheimer's or mild cognitive impaired (MCI) patients.
Immunotherapy Used to Reduce Levels of Amyloid Beta Proteins
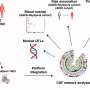
Reducing amyloid-beta proteins through immunotherapy has shown benefits in previous mice studies. However, new research led by Martin Sadowski, MD, an associate professor of neurology, psychiatry, and biochemistry and molecular pharmacology, at NYU Langone, suggests that more studies are needed to reduce potential risks associated with this possible approach to treatment.
Genetic variants of apolipoprotein E (APOE), a specific gene mapped to chromosome 19, is among the most significant factors predicting susceptibility to Alzheimer's disease caused by buildup of amyloid beta plaques. There are three variants of APOE: APOE ε2, APOE ε3 and APOE ε 4. Previous research has shown that variant APOE ε4 significantly increases Alzheimer's disease risk, while APOE ε 2 reduces risk for the disease; the impact of APOEε3 is less known. An estimated 20 percent of people carry the risk-increasing APOE ε4 genetic variant.
Immunotherapy has been studied to target amyloid beta plaque build-up, but previous studies have shown that APOE ε4 subjects are prone to develop specific adverse effects called Amyloid Related Imaging Abnormalities (ARIA), which include micro-hemorrhages, or bleeding of the brain, when given this treatment.
In the new study, Sadowski and colleagues examined the effect of amyloid beta immunotherapy in Alzheimer's transgenic mice, engineered to have each of the three genetic variants of human APOE. They found a greater effect of immunotherapy on reducing the load of amyloid-beta deposits in mice expressing the APOE ε 4 variant than in mice expressing other APOE variants. Clearance of amyloid-beta deposits was associated with increased activation of brain cells called microglia, which uptake and digest amyloid beta. Microglia activation was much greater in mice expressing the APOE ε 4 variant than in mice expressing other APOE variants, which may cause harmful effect of stimulated microglia on the brain.
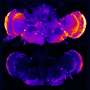
The researchers also discovered that in Alzheimer transgenic mice, APOE variants have different effects on amyloid beta deposition in the walls of brain vessels, with the APOE ε 4 promoting deposition of amyloid beta in large vessels, while APO ε2 lead to more amyloid beta deposition in the brain's micro-vessels.
Immunotherapy cleared brain vessels of amyloid beta, but it was associated with increased incidence of brain bleeding. The greatest incidence of brain bleeds was noticed in APOE ε 2 mice while the lowest was found in APOE ε 3 mice.
These new findings provide previously unknown information that may aid in development of immunologic treatments for Alzheimer's.
"Our study identifies the previously underappreciated risk-promoting effect of APOE ε 2 and the protective effect of APOE ε 3 on the incidence of brain bleeding associated with amyloid-beta immunization," says Sadowski. "This is an important observation in a mouse model that may influence how immunotherapy treatments, such as vaccines, for Alzheimer's are developed and one day tested in humans."