Researchers find key to muscle regeneration

Saint Louis University researchers report in Molecular Metabolism new findings that the nuclear receptor REV-ERB appears to play a key role in muscle regeneration, suggesting the receptor may be a good target for new drugs to treat a variety of muscle disorders and injuries.
Colin Flaveny, Ph.D. assistant professor of pharmacology and physiology and Thomas Burris, Ph.D., chair of pharmacology and physiology at Saint Louis University, focus their work on identifying natural hormones that regulate nuclear receptors and then developing synthetic compounds to target these receptors in order to develop drugs to treat diseases.
Earlier this year, Burris published findings showing that a nuclear receptor called REV-ERB is involved in lowering LDL cholesterol. He previously studied REV-ERB's role in regulating mammals' internal clocks.
Now teaming up, Flaveny and Burris are uncovering REV-ERB's role in muscle regeneration.
"REV-ERB is an interesting nuclear receptor that helps coordinate our metabolism with our daily routine," Flaveny said. "We're studying the protein to see if turning its activity up or down can influence the way muscle regenerates after injury or illness."
Skeletal muscle comprises 40 to 50 percent of our total body mass and is essential for postural support, locomotion and breathing. With a high capacity for regeneration, skeletal muscle normally maintains muscle mass and function in response to minor injuries and normal wear and tear without much trouble.
However, in cases of traumatic injury or illnesses like congestive heart failure, chronic obstructive pulmonary disease, severe burns, cancer and HIV infection, the body's natural muscle regeneration may not be able to keep up and the loss of skeletal muscle mass and strength is common.
When injuries are severe - with more than 20 percent loss of muscle mass - normal muscle regeneration often cannot keep pace with the regenerative demands. In this scenario, the loss of skeletal muscle mass can trigger widespread fibrosis and loss of muscle function.
Eventually, muscle regeneration may become unable to keep up, even with assistance through dietary interventions, anabolic steroids or non-steroidal anti-inflammatories (NSAIDs). The use of anabolic steroids and NSAIDs are accompanied by severe side effects that may further reduce quality of life. Often, pharmacological interventions fail to stem long-term decline in quality of life or enhance survival for those with degenerative muscle tissue diseases.
"Identifying new means of accelerating muscle regeneration has proved a daunting challenge," Burris said. "Therefore understanding the underlying mechanisms that regulate muscle cell regeneration and coordinate regenerative repair could provide future therapeutic options for stymieing the loss of muscle function in the traumatically injured."
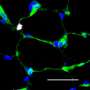
A simplified version of muscle cells' life-cycle looks like this: muscle stem cells produce myoblasts that will either reproduce (proliferate) or form muscle tissue (differentiate). Successful regeneration of skeletal muscle after traumatic injury depends on the replenishment of muscle fibers through elevated myoblast proliferation and differentiation.
Scientists were fascinated to see that REV-ERB appears to play different roles for different stages of muscle tissue development. A decline in expression of REV-ERB precedes myoblast differentiation. Conversely, an increase in REV-ERB expression is involved in the regulation of mitochondrial and metabolic function in fully differentiated skeletal muscle.
The research team identified a mechanism through which REV-ERB may regulate gene expression pre and post muscle differentiation. They show that REV-ERB is a regulator of muscle differentiation that can be targeted to stimulate muscle regeneration and may be useful in treating numerous muscle diseases, including muscular dystrophy, sarcopenia and cachexia, in addition to acute injury.
"We demonstrate that REV-ERB can stimulate muscle regeneration upon acute muscle injury in an animal model," Burris said. "Our findings reveal that REV-ERB may be a potent therapeutic target for the treatment of a myriad of muscular disorders."
More information: Ryan D. Welch et al, Rev-Erb co-regulates muscle regeneration via tethered interaction with the NF-Y cistrome, Molecular Metabolism (2017). DOI: 10.1016/j.molmet.2017.05.001