Calcium lets T cells use sugar to multiply and fight infection
A calcium signal controls whether immune cells can use the nutrients needed to fuel their multiplication into a cellular army designed to fight invading viruses.
This is the finding of a study in human cells and mice led by researchers at NYU School of Medicine and published online October 10 in Immunity.
The study results concern the precise and massive immune counterattack by T cells in response to viral infection. When this type of white blood cell is turned on by an invader, it divides and multiples into an army of clones primed specifically to attack that invader.
The current study found that whether or not T cells can use energy from blood sugar (glucose) to multiply depends on calcium flow into cells, a mechanism not previously recognized, say the authors.
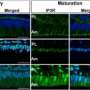
Upon receiving the right signal - which in this case is the recognition of virus particles - T cells open channels in their outer membranes, letting calcium rush in to activate the protein NFAT, a transcription factor that turns on genes, say the researchers. Specifically, the new study shows that a particular type of calcium influx - store-operated calcium entry (SOCE) - controls the activation of NFAT and its ability to turn on genes that control the uptake and breakdown of glucose.
"Our results argue that SOCE, in cooperation with the enzyme calcineurin, regulates the conversion of glucose into cellular energy and building blocks needed for T cell proliferation," says senior author Stefan Feske, MD, an associate professor in the Department of Pathology's Experimental Pathology Program at NYU School of Medicine.
The study results also are relevant to autoimmune diseases, such as rheumatoid arthritis or lupus, where an oversensitive immune system becomes prone to attack the body's own tissues. Treatments for these diseases seek to dial down the immune response, as do treatments meant to keep the immune system from attacking and rejecting transplanted organs.
Two drugs widely used in clinical practice tacrolimus and cyclosporin A, suppress the immune system to counter both autoimmunity and transplant rejection by preventing the enzyme calcineurin from activating NFAT. The new study by Feske and colleagues explains for the first time that the effect of tacrolimus on the calcineurin/NFAT pathway shuts down the ability of T cells to use glucose.
"Based on these results, we will look closer in future experiments at how SOCE regulates the metabolism of immune cells during autoimmune diseases, as well as immune responses against cancer," says co-first author Martin Vaeth, PhD, a postdoctoral researcher in Feske's laboratory.
It is well established that, during SOCE, calcium enters T cells through calcium release-activated (CRAC) channels, say the authors. The research team found that mice genetically engineered to lack the genes for correctly building these channels were less able to fight viral infections. When researchers artificially inserted glucose-handling genes that are regulated by SOCE back into T cells, it restored their ability to proliferate and fight infection.
Researchers also isolated T cells from human patients with CRAC channel deficiencies, very rare diseases caused by changes to the genes that code for the channels. Such patients are extremely susceptible to infections early in life. The new study found that the T cells in such patients did not multiply (proliferate) and failed to take up and use glucose. They also found that NFAT action was required to flip the "metabolic switch" that drives T cell proliferation.
"These results are timely because the field is currently exploring whether a drug class called CRAC channel inhibitors can be used safely against autoimmune and inflammatory diseases in human patients, and our study fills in key details on some of the relevant mechanisms," says Vaeth.