Fred Hutch researchers engineer complex immunotherapy that may target relapsing leukemia

Oct. 25, 2017 - Researchers at Fred Hutchinson Cancer Research Center and the University of Washington have developed a novel way to genetically engineer T cells that may be effective for treating and preventing leukemia relapse.
The findings, published online in the journal Blood, provide the basis for launching a first-in-human clinical trial of this new immunotherapy, which relies on engineered T-cell receptors, or TCRs. This immunotherapy represents a different method of genetic engineering than the CAR T-cell therapies that were recently approved by the U.S. Food and Drug Administration.
Relapse occurs in about one-third of patients with acute leukemia who undergo stem cell transplantation to rebuild cancer-free blood cells, and more than 90 percent of these patients die after an average survival of about four months.
"New therapies are desperately needed to prevent and treat relapse of leukemia in patients who have undergone hematopoietic stem cell transplantation," said pediatric oncologist Dr. Marie Bleakley, the paper's senior author, who is a member of Fred Hutch's Clinical Research Division.
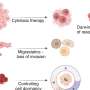
T cells, a linchpin of the immune system, have a variety of molecules on their surface, known as receptors, that recognize cells that are foreign or diseased and kill them. To boost the immune system's ability to recognize and attack these "invaders," researchers may transfer genes for a tumor-specific T-cell receptor into the T cells collected from a patient's transplant donor.
In this work, Bleakley and colleagues exploited a specific "minor histocompatibility antigen," or minor H antigen, found on the surface of leukemia cells in some patients. Using this group of antigens as targets is being re-examined now that the basic principles of cancer immunotherapy are better understood and potent T-cell immunotherapy is a clinical reality. Because these antigens are expressed predominantly on blood-forming cells, targeting them could provide a potent and selective anti-leukemia treatment with little risk to other cells.
TCR therapy differs from CAR T-cell therapy in that the latter involves creating receptors that are not found in nature. The former occurs naturally in humans, though the receptors we have can vary. While CAR T-cell therapies are known to be effective in treating B-cell acute lymphoblastic leukemia, or ALL, it has not yet been successful in acute myeloid leukemia or T-cell ALL.
Bleakley's team broke new ground by identifying T-cell receptors that were especially potent in their targeting of a minor H antigen found on the surface of leukemia cells. Using these genetic blueprints, they then were able to extract these receptors from select blood samples provided by donors. Next, they inserted these receptors into T cells from donors for patients who could perhaps benefit from having such "supercharged" T cells to seek and destroy cancer cells with the targeted antigen.
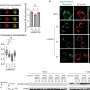
Although no patients have yet received these TCRs, the engineered T cells efficiently and specifically killed target cells in laboratory tests.
"T-cell receptors isolated from minor H antigen-specific T cells represent an untapped resource for developing targeted T-cell immunotherapy to manage leukemia relapse," Bleakley said, adding that the construct used in this study could serve as a prototype for others targeting similar antigens. Her research team has established a new technique to discover antigens that may be exploited as targets and has identified and characterized five novel minor H antigens.
Bleakley is aiming to launch a Phase 1 clinical trial in December 2017. If results from the lab are borne out in clinical trials, this form of adoptive T-cell therapy could join a growing immune-based arsenal. Fred Hutch researchers and clinicians are pioneers in the development of a variety of T-cell therapies for blood-related and other cancers.
More information: Robson G. Dossa et al, Development of T cell immunotherapy for hematopoietic stem cell transplantation recipients at risk of leukemia relapse, Blood (2017). DOI: 10.1182/blood-2017-07-791608