Introducing titin, the protein that rules our hearts

Although scientists have long speculated that a protein named titin measures thick filaments—the proteins that make muscles contract—no one has been able to provide evidence to support their theories.
No one, that is, until a team of researchers in the University of Arizona's Department of Cellular and Molecular Medicine took on the case. In a recent study published in Nature Communications, the team presented definitive proof that titin acts as a molecular ruler for the muscle's thick filament.
Throughout the muscles of the heart and body, the thick filaments have precise, uniform lengths.
"Functionally and clinically, it is very important to regulate the thick filament precisely, otherwise muscles would not function well," said Henk Granzier, senior author of the study and professor of molecular and cellular medicine. "Biologists have always wondered what makes them so precisely structured."
Studying mice with certain mutations in the gene encoding the blueprint for the titin protein, the researchers found that when titin was shortened, so were the thick filaments, resulting in weakened muscles and dilated cardiomyopathy, a condition that leads to heart failure.
"We genetically engineered a mouse that doesn't have normal titin," said Granzier, a member of the BIO5 Institute. "It has a piece from titin deleted. Since titin is involved in somehow regulating the thick filament, then you expect if you make titin shorter, the thick filament length will be altered as well. And, lo and behold, that is the case."
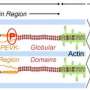
A typical protein is made of a few hundred amino acids linked in a chain. Though still microscopically small, titin is gigantic compared to other proteins. Comprised of more than 30,000 amino acids, the supersize protein is made from super-repeated structures. The amino acid super-repeats of titin are like tick marks on a yardstick, measuring out uniform sections of the thick filament.
By altering the gene for titin, Granzier's team was able to make a mouse whose titin was missing several of its super-repeats.
The resulting mice showed symptoms of dilated cardiomyopathy, or DCM. This condition stretches out the muscle in the heart and prevents it from pumping efficiently. While the heart still contracts, the muscle is weak, so each contraction only moves a fraction of the blood pumped by a normal heart.
In humans, the most frequent cause of DCM is a mutation in titin that shortens the protein. DCM affects 1 in 500 people, and often patients must undergo a heart transplant to survive. Understanding the cause of the condition can better arm researchers as they search for novel ways to combat it.
Methods of Discovery
"In science, if you want to do conclusive work, you have to test your hypothesis multiple ways and get consistent results," Granzier said. This meant that any abnormalities detected in the genetically engineered mice had to be deeply investigated.
After testing the strength of the mice, his team removed the muscles of interest to inspect them in a setting they controlled, instead of a setting controlled by the complex biological systems of the mice. To make certain that differences in the mice were caused by titin, the muscle tissues were observed in vitro by removing them from the animals and artificially stimulating them to show that they produced less force. In the body, muscles contract when activated by calcium; under the microscope, flooding the isolated muscle tissue with a calcium solution mimics this activation process.
Using ultrasound, the team showed that the hearts of the engineered mice were larger than those of mice with normal titin. The next step was to investigate how this enlargement weakened the heart.
How Titin Controls Strength
"The sarcomere is the smallest muscle unit you can tease out and still have all the properties of muscle: force development and shortening," Granzier said.
The sarcomeres are linked end-to-end in a chain that spans the entirety of a muscle. Just as a strong chain is made from links that are well made and uniform, sarcomere health and uniformity is vital to muscle strength.
Each sarcomere is comprised of three filaments: thick, thin and titin filaments. The motor driving contraction—myosin—is held at precise points within the thick filament, and it pulls the thick filament across the thin filament, causing muscle contraction. Muscles are strongest when the thick and thin filaments overlap at an optimal length—around 2 micrometers, or one ten-thousandth of an inch. Stretch out the muscle, and the filaments cannot reach the point of optimum overlap, so the muscle is weakened. Overstretch the muscle, and the filaments do not overlap at all; the muscle cannot exert any force.
When titin is mutated and short, the resulting shortened fibers cannot reach the optimal point of overlap, and the muscle cannot exert much force. The muscle is further weakened because shorter thick filaments cannot hold the optimal number of myosin motors. The thin and thick filaments do not overlap properly nor contract effectively when the thick filament is short.
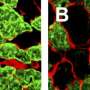
Paula Tonino and Balazs Kiss, lead authors of the study and scientific investigators in Granzier's lab, observed the muscle fibers under electron and super-resolution microscopes. They determined that in the muscles of engineered mice, not only were the thick filaments shortened, but also that they were shortened by precise, uniform lengths that corresponded to the size of the super-repeats removed from titin.
"We showed that titin is the regulator of the thick filament," Granzier said, confirming that titin determines the strength of muscles and health of hearts.
"You might say titin rules."