Critical toxic species behind Parkinson's disease is glimpsed at work for the first time
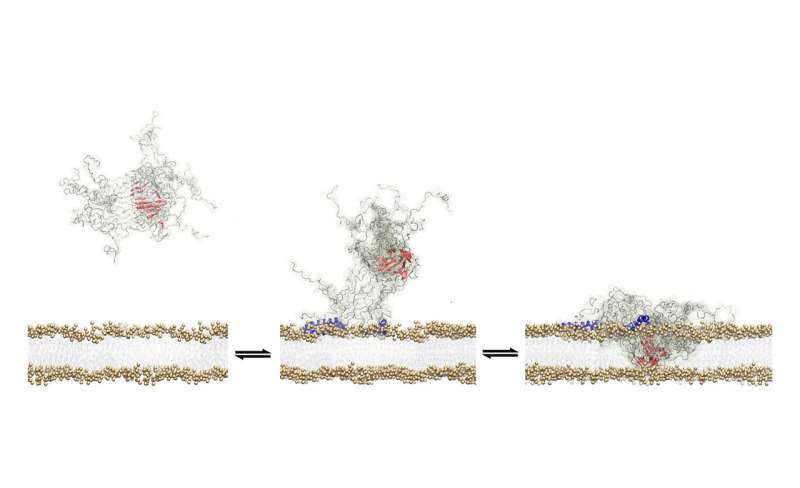
Researchers have glimpsed how the toxic protein clusters that are associated with Parkinson's Disease disrupt the membranes of healthy brain cells, creating defects in the cell walls and eventually causing a series of events that induce neuronal death.
The study examined what are known as toxic oligomers, clusters of protein molecules that emerge when individual proteins misfold and clump together. In the case of Parkinson's Disease, the protein involved is called alpha synuclein, which when it is functioning normally plays an important part in signalling in the brain.
The formation and spread of these clusters is thought to be a key component of the underlying molecular mechanisms of this progressive illness. Understanding how they enter and damage cells presents an opportunity to develop new and more effective treatments. But until now, studying how they damage brain cells has been extremely difficult as they are typically unstable. Shortly after forming they either fall apart, or assemble into larger structures that are less damaging to individual cells.
In the new study, academics were able to stabilise oligomers long enough to examine how they damage brain cell walls in unprecedented detail. They identified a specific feature of the oligomer which allows it to latch on to the cell wall, and a "structural core", which then breaks through.
The research was carried out by an international team of scientists from the UK, Italy and Spain, led by Professor Christopher Dobson, at the University of Cambridge, and Dr Alfonso De Simone, from Imperial College London.
"It is a common property of oligomers that they can damage brain cells once they bind to the surface," De Simone explained. "It is a bit like if you put a piece of extremely hot metal on to a plastic surface. In a fairly short space of time it will burn a hole through the plastic. The oligomer does something similar when it comes into contact with the cell membrane, and this disrupts the integrity of the membrane, which is the key step in the mechanisms leading to the death of the neuron."
Dr Giuliana Fusco, a postdoctoral researcher at St John's College, Cambridge who carried out much of the experimental work for the study while working towards her PhD, said: "Just having this information doesn't mean that we can now go and make a drug, but obviously if we can understand why these clumps of proteins behave the way they do, we can make faster scientific progress towards treating Parkinson's Disease. It means we can take a more rational approach to drug discovery."
Toxic oligomers form at an early stage in the series of events that lead to Parkinson's Disease, which are believed to begin when alpha synuclein proteins malfunction and begin to stick together. Their emergence is lethal to neuronal function in this context. Once the oligomers have formed, they disperse, and allow the initial toxicity to spread to other cells.
In the study, researchers examined samples in the lab of both toxic and non-toxic forms of oligomeric alpha synuclein oligomers using solid state nuclear magnetic resonance spectroscopy (SSNMR). Recent developments in this technology enabled them to study the oligomers in a level of detail that was not previously possible. The research team characterised different features of the structures, and then studied how these various properties influenced their interactions with sample brain cells taken from rats, as well as additional cells taken from human brain tumours.
The study's results could, in particular, inform the identification of molecules that can attack these damaging toxins and hence limit their impact. In October this year, the University of Cambridge launched a new Centre for Misfolding Diseases, based in the Department of Chemistry, and geared towards developing therapeutic strategies for a range of conditions including Parkinson's Disease. Much of its work will build on studies which, like this one, enhance scientific understanding of the fundamental processes that underlie neurodegeneration. From this, molecular "candidates" that might be used in future drugs can be both identified and, if necessary, optimised to target disease.
Professor Dobson, who is both Master of St John's College, Cambridge, and a Director of the Centre for Misfolding Diseases, said: "One of the really exciting things about this work is that not only has it been possible to define the structure of the critical pathogenic species in a neurodegenerative disorder, but we have also managed to propose a mechanism for its toxicity, providing vital clues for pursuing rational therapeutic strategies.."
The report, Structural basis of membrane disruption and cellular toxicity by a-synuclein oligomers, is published in the journal Science.
More information: "Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers" Science (2017). science.sciencemag.org/cgi/doi … 1126/science.aan6160