Identifying the dangers of chronic stress on multiple sclerosis
![Credit: Hokkaido University Identifying the dangers of chronic stress on multiple sclerosis [Asia Research News 2018 feature]](https://scx1.b-cdn.net/csz/news/800a/2018/identifyingt.jpg)
New research reveals how chronic stress and tiny brain inflammations cause fatal gut failure in a multiple sclerosis mouse model.
A newly discovered nerve pathway facilitates fatal gut failure in a multiple sclerosis (MS) mouse model placed under chronic stress, Hokkaido University researchers report in the journal eLife. The findings could provide a new therapeutic strategy for MS, an intractable, currently untreatable disease.
MS affects an estimated 2.5 million people worldwide and causes motor dysfunction, impaired vision and gut failure. It is an autoimmune condition of the central nervous system mediated by immune cells called autoreactive CD4+ T cells.
These pathogenic CD4+ T cells can be used to induce an MS-like disease in research mice. In previous studies using these mouse models, Masaaki Murakami of Hokkaido University and his colleagues found that autoreactive CD4+ T cells cross the blood-brain barrier at specific sites causing brain and spinal cord inflammation.
In the present study, the team and their collaborators in Japan and Germany investigated the possible relationships between micro-inflammation in the brain, chronic stress and stress-related organ failure.
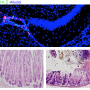
![Micro-inflammation developed at specific sites in the brain (top). Pathological analysis showed damage to stomach tissues (bottom right) compared to mice not placed under stressful conditions (bottom left). Credit: Arima Y. et al., eLife Identifying the dangers of chronic stress on multiple sclerosis [Asia Research News 2018 feature]](https://scx1.b-cdn.net/csz/news/800a/2018/1-identifyingt.jpg)
They put healthy mice under stress by disturbing their sleep or by rearing them on wet bedding. Transferring pathogenic CD4+ T cells to the mice under stress caused severe symptoms such as gut failure and even sudden death. Cell micro-inflammation developed at specific sites in the brain (top). Pathological analysis showed damage to stomach tissues (bottom right) compared to mice not placed under stressful conditions (bottom left). transfer or stress alone did not cause these symptoms. Subsequent investigations revealed a complex nerve-related mechanism behind this process.
The injected pathogenic CD4+ T cells accumulated around blood vessels in two specific sites at the centre of the brains of stressed mice. Micro-inflammation developed around specific blood vessels, and the inflamed sites then released a small molecule, called ATP, that switched on a nerve pathway that is normally turned
off. This switch led to gut dysfunctions, bleeding and failure. Also, the bleeding led to increased levels of potassium in the blood, which is a factor that can lead to heart failure.
The team was able to prevent gut failure by suppressing inflammation in the brain or blocking nerve pathways from the brain to the gut. The results suggest tiny areas of inflammation around some specific vessels in the brain are a risk factor for organ dysfunction, including severe gut and heart failure. Micro-inflammation in the brain is known to happen in various diseases, including multiple sclerosis, Alzheimer's disease and Parkinson's disease.
"These results demonstrate a direct link between brain micro-inflammation and fatal gastrointestinal diseases via the establishment of a new neural pathway under stress," says Murakami. "We plan to further investigate other possible connections between brain micro-inflammations and organ dysfunctions, including those within the brain itself."