There is more going on in myotonic dystrophy type 1 than just alternative splicing
Myotonic dystrophy type 1 (DM1) is the most common adult-onset muscular dystrophy that affects multiple organ systems. People with this condition develop progressive muscle wasting and weakness in their lower legs, hands, neck and face. Their muscles feel stiff and tight, causing them to be slow to relax certain muscles and therefore have difficulty releasing the hand from a handshake or a doorknob. In addition, people with this condition may have fatigue, muscle pain, difficulty swallowing, cataracts, irregularities in their heartbeat and respiratory complications. In his laboratory at Baylor College of Medicine, Dr. Thomas A. Cooper is leading the way to better understand this rare but devastating condition.
"Muscle wasting in this disease, which happens over decades, is responsible for the death of 60 percent of the patients," said Cooper, who is professor of pathology and immunology, of molecular and cellular biology and of molecular physiology and biophysics at Baylor College of Medicine. "In this study we wanted to develop a novel model of the disease that would allow us to study muscle wasting in more detail."
DM1 is caused by a striking expansion of three-letter repeats (CTG) in the DMPK gene. While the unaffected population carries 5 to 37 repeats, people with the condition have 50 to 3000 repeats. The RNA transcripts containing the CTG repeat expansion accumulate in the cell nucleus. This disturbs the normal cellular processing and distribution of molecules, such as muscleblind-like (MBNL) proteins, and induces up-regulation of others, such as the CELF1 protein. These alterations result in abnormal alternative splicing, which is thought to play a central role in the development of DM1. However, how these changes triggered by the expansion of the CTG repeat lead to muscle wasting still is not completely understood.
"We think that the current animal models of DM1 do not provide researchers with a complete and practical tool to investigate the mechanisms involved in muscle loss," said Dr. Ginny Morriss, postdoctoral associate in the Cooper lab and the first author of this work. "This disease has many different components. Current animal models have some of the molecular components, but the physiological components, what's happening to the tissue, are mostly missing. We wanted to develop a mouse model of DM1 that clearly showed muscle loss and to implement a strategy that would allow us to study the pathways involved in muscle wasting."
A mouse model of reversible DM1
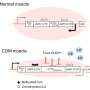
The researchers genetically engineered a skeletal muscle-specific mouse model of DM1 that allowed them to induce the development of the disease at will. When induced, the mice expressed 960 CUG repeats of a particular region of the human DMPK gene and the RNA transcripts containing the CUG repeat expansion accumulated inside the cell nucleus triggering the chain of events that resulted in progressive muscle wasting. When the researchers 'turned off' the expression of the 960 CUG repeats, RNA accumulation and muscle loss progressively reverted.
In this model, the researchers saw alternative splicing that was consistent with findings in previous studies that correlated it with muscle weakness. They also validated signaling pathway changes that had been previously found by others. Importantly, they saw signaling pathway changes that had not been described before. These new changes stratified with how severe muscle wasting was in the mice, showing a clear association between specific signaling pathways and muscle loss.
"We validated the upregulation of the activity of protein AMPK-alpha, which had been shown previously by another group in another model. AMPK-alpha regulates the way the muscles metabolize and function," Morriss said. "One of the new changes we discovered in our model was the dramatic reduction of signaling activity mediated by PDGFR-beta, which is involved in energy metabolism pathways."
In addition, Cooper, Morriss and their colleagues found a connection with the human condition. They analyzed human tissue samples from patients and unaffected individuals and found in the patients the same signaling pathway changes they had found in their mouse model.
"The field has been focusing on alternative splicing. But, one of the things our findings tell us is that, although many of the characteristics of the disease result from alternative splicing defects, in addition there are other mechanisms at play and therefore other potential targets to treat this disease. There is more going on here than just alternative splicing," said Cooper, who also is the S. Donald Greenberg and R. Clarence and Irene H. Fulbright Professor and a member of the Dan L Duncan Comprehensive Cancer Center at Baylor.
"Now we have a mouse model in which we can test mechanisms involved in the disease. Because we made our model reversible, we can use it to test hypotheses about how the repeats cause the characteristics of the disease. We can systematically test each one of those hypothesis independently in our model blocking each signaling event specifically and determining how much that affects the disease. We can in this way determine how much each of the disease components, signaling pathways and alternative splicing, contribute to the disease," Cooper said.
More information: Ginny R Morriss et al, Mechanisms of skeletal muscle wasting in a mouse model for myotonic dystrophy type 1, Human Molecular Genetics (2018). DOI: 10.1093/hmg/ddy192