Traffic jams in the brain
Traffic jams can also occur in the brain, and they can be damaging. Researchers at Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) have been able to prove that disrupted transportation routes in nerve cells are a significant cause of Parkinson's disease.
Nerve fibres give nerve cells their characteristic long shape. Measuring up to one metre in length, they form the contact points to other nerve cells. In order to carry out the important task of communicating with other nerve cells, the fine branches of these nerve fibres and their ends, called synapses, must be regularly supplied with energy from the cell body. If this energy supply is interrupted, the synapses are destroyed. Connections between nerve cells are then disrupted, which can lead to the cells dying off. This process is typical for the development of brain disorders such as Parkinson's disease.
It is unclear which mechanisms are responsible for the loss of nerve cells in Parkinson's. Researchers at FAU, led by Dr. Iryna Prots and Prof. Dr. Beate Winner from the Department of Stem Cell Biology, in conjunction with collaborators, have now demonstrated that a type of 'traffic jam' in the nerve cells could be the cause.
The researchers discovered that the traffic jam is triggered by a protein called alpha-synuclein, which is also found in healthy nerve cells. In abnormal nerve cells, the protein forms deposits, or even lumps, leading to a delay, disrupting the energy supply of the nerve fibres, and ultimately damaging the synapses.
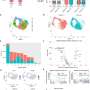
The researchers were also able to demonstrate this mechanism in cell cultures taken from patients with Parkinson's. A small skin sample was taken from affected patients. These skin cells were then converted into stem cells, which can be developed into any type of cell, and in this case, into nerve cells.
In initial trials, the researchers succeeded in suppressing the formation of lumps of alpha-synuclein, thus improving the transportation of information in the nerve fibres. However, the substance they used has not yet passed clinical trials. Nevertheless, the lead author of the study, Dr. Iryna Prots, says, "Our findings mean we can improve our understanding of the mechanisms that cause Parkinson's and push forward new strategies for treatment during the progression of the disease."
More information: Iryna Prots et al, α-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1713129115