Blocking pro-fibrosis pathway may improve immunotherapy of metastatic breast cancer
A Massachusetts General Hospital (MGH) research team has found that the overgrowth of connective called fibrosis may block the effectiveness of immunotherapies against metastatic breast cancer. Their report published in PNAS also finds that plerixafor, a drug approved to mobilize blood system stem cells in the treatment of lymphoma and multiple myeloma patients, can reduce fibrosis in both primary and metastatic breast tumors and improve response to immunotherapy in mouse models.
"Improving the survival of patients with metastatic breast cancer remains a significant challenge; and while immunotherapy, which harnesses the power of the immune system against cancer, has shown some promise, it remains less effective against metastatic breast cancer," says Ivy Chen, Ph.D., post-doctoral fellow in the Edwin L. Steele Laboratories for Tumor Biology in the MGH Department of Radiation Oncology and lead author of the PNAS report. "While fibrosis has been extensively studied in primary breast tumors, little is known about the level of fibrosis and its role in immunosuppression in metastatic lesions."
A key focus of research in the Steele Labs, directed by Rakesh Jain, Ph.D., senior author of the PNAS report, has been understanding how physical features of tumors can impede the effectiveness of cancer therapies. Many treatment-resistant tumors are what is called desmoplastic—characterized by an overgrowth of connective tissue, which can block or even repel cancer-killing T cells from entering tumors, as well as inhibiting the effectiveness of traditional anti-cancer therapies. Studies in other types of cancer have identified a role for the CXCL12/CXCR4 signaling pathway, known to regulate immune cells, in resistance to immunotherapies.
In their investigation of this potentially important signaling pathway in metastatic breast cancer, the MGH team found the following:
- Analysis using The Cancer Genome Atlas database identified a role for CXCL12/CXCR4 signaling in the exclusion of cancer-fighting CD8 T cells from human breast cancers.
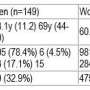
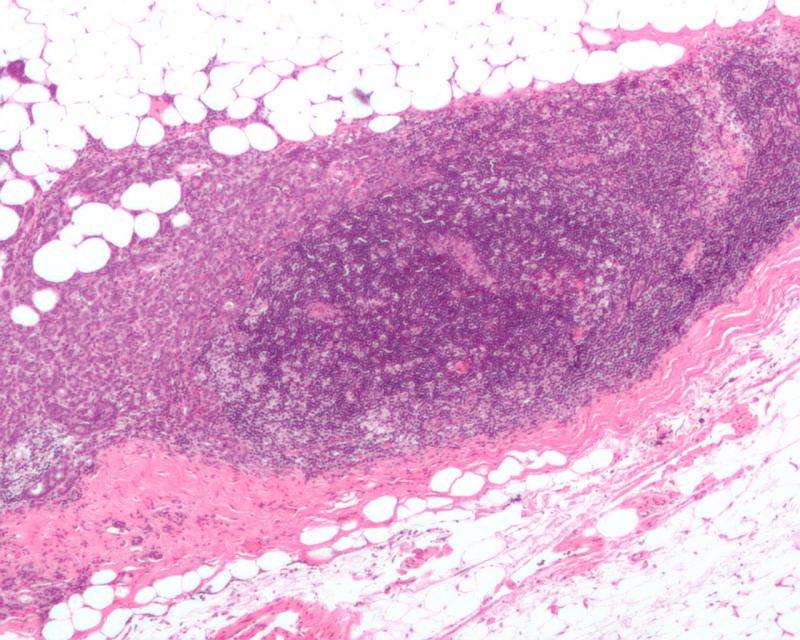
- Examination of paired biopsies of primary and metastatic breast tumors from the same patients revealed that CXCR4 expression correlated with high levels of desmoplasia and expression of immunosuppressive proteins in all subtypes of breast cancer.
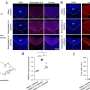
- Experiments using mouse models of metastatic breast cancer showed that plerixafor—which inhibits CXCR4—reduced desmoplasia and the expression of profibrotic and immunosuppressive genes.
- Silencing the CXCR4 gene in a mouse model revealed that these immunosuppressive effects are dependent on CXCR4 signaling in fibroblasts, cells that produce fibrosis and promote immunosuppression.
- CXCR4 inhibition improved the infiltration of T cells into breast cancer metastases and decreased the formation of spontaneous metastases in the lung, one of the most common sites for metastatic breast cancer.
- CXCR4 blockade sensitized three mouse models to immune checkpoint blockers, a form of immunotherapy that has transformed the treatment of a number of malignancies but not breast cancer.
Overall this study was the first to show that primary and metastatic breast tumors are highly fibrotic, and identified a correlation between CXCR4 expression, fibrosis and immunosuppression in primary and metastatic breast cancer. Significantly, it showed that CXCR4 inhibition may enhance the effectiveness of immunotherapy for metastatic breast cancer.
"Our findings suggest that fibrosis-targeting drugs, such as plerixafor, may be able to benefit breast cancer patients with late-stage, metastatic disease." says Jain, the Cook Professor of Radiation Oncology at Harvard Medical School "Most importantly, because not all metastatic breast cancers respond to immunotherapy, CXCR4 inhibition may improve response to those treatments by reducing fibrosis and immunosuppression. Our findings provide the necessary data and rationale for clinical trials to test the efficacy of combining CXCR4 inhibition using plerixafor, an FDA-approved drug for other indications, with immune checkpoint blockade for metastatic breast cancer patients."
Co-author Robert Langer, ScD, Koch Institute Professor at Massachusetts Institute of Technology, adds, "With more than 40,000 women dying from breast cancer annually in the U.S. alone, this work provides a rapidly translatable strategy and potential hope for these patients."
More information: Ivy X. Chen el al., "Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer," PNAS (2019). www.pnas.org/cgi/doi/10.1073/pnas.1815515116