Brake on immune activity identified, raising new possibilities for anticancer therapy
The immune system is like a carefully regulated machine, complete with its own built-in "brakes" that prevent it from overreacting and causing excess inflammation in otherwise healthy tissues. This preventative safety net, however, is highly vulnerable, particularly in cancer, where tumor cells step on the brakes constantly, because doing so allows the tumor cells to escape immune detection.
Several molecules that act as natural brakes on immune activity have been discovered, which has opened the door to immunotherapy—a potentially highly effective way of leveraging the immune system to attack cancer cells. For immunotherapy to reach its full potential in human patients, however, more must be learned about factors driving cancer immunity.
Now, researchers at the Lewis Katz School of Medicine at Temple University (LKSOM) and Fox Chase Cancer Center show for the first time that a molecule called EGR4 -known mainly for its role in male fertility—serves as a critical brake on immune activation. The new study, published online March 25 in the journal EMBO Reports, shows that taking EGR4 away—effectively releasing the brake—promotes the activation of so-called killer T cells, which infiltrate and attack tumors and thereby boost anticancer immunity.
"Other early growth response proteins, or EGRs, are important to T cell activity, but whether EGR4 also has a role in immunity has been largely overlooked," explained Jonathan Soboloff, Ph.D., Professor of Medical Genetics and Molecular Biochemistry at the Fels Institute for Cancer Research and Molecular Biology at LKSOM. "Our study reveals a new side to the importance of EGR4."
Dr. Soboloff's team examined the influence of EGR4 expression in immune cells in collaboration with Dietmar J. Kappes, Ph.D., Professor of Blood Cell Development and Cancer at Fox Chase Cancer Center.
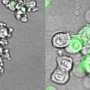
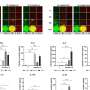
In initial experiments, the researchers found that T cell activation is associated with EGR4 upregulation. They then showed that knocking-out, or eliminating, EGR4 from immune cells results in a dramatic increase in calcium signaling and expansion of T helper type 1 (Th1) cell populations. Th1 cells, in response to the presence of foreign entities, including tumor cells, activate cytotoxic, or killer, T cells, which then wipe out the invader.
"We know from our previous work that T cells control calcium signaling and that when intracellular calcium levels are elevated, calcium signaling can drive T cell activation," Dr. Soboloff said.
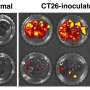
The Soboloff and Kappes labs next studied the functional importance of EGR4 in cancer immunity by utilizing an adoptive mouse model of melanoma in which some host animals lacked EGR4 expression. Compared to mice with typical EGR4 levels, EGR4 knockout animals showed evidence of expanded populations of Th1 cells and enhanced anticancer immunity. In particular, EGR4 knockout mice had reduced lung tumor burden and fewer metastases than mice with normal EGR4 expression.
In future work, the Soboloff and Kappes groups plan to further explore strategies for EGR4 targeting. The development of an agent to target EGR4 specifically may be difficult, due to the diverse actions of EGR pathways. "But eliminating EGR4 specifically from a patient's T cells, and then putting those cells back into the patient, may be a viable immunotherapeutic approach," Dr. Kappes said.