In a cell-eat-cell world calcium ions activate 'eat-me' signal in necrotic cells
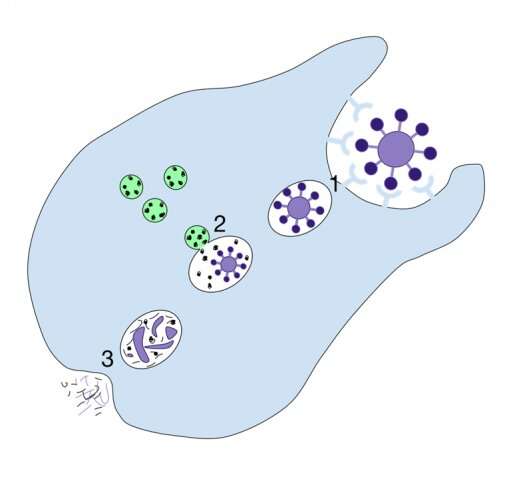
Just as people keep their houses clean and clutter under control, a crew of cells in the body is in charge of clearing the waste the body generates, including dying cells. The housekeeping cells remove unwanted material by a process called phagocytosis, which literally means 'eating cells.' The housekeepers engulf and ingest the dying cells and break them down to effectively eliminate them.
"Phagocytosis is very important for the body's health," said Dr. Zheng Zhou, whose lab at Baylor College of Medicine has been studying phagocytosis for many years and provided key new insights into this essential process. "When this cell-eat-cell process fails, the dying cells will lose their integrity, break down and release their content into the surrounding tissues. Dumping the cell content would cause direct tissue damage and trigger inflammatory and autoimmune responses. If the dying cells are infected by a virus, releasing the cellular content would spread the infection."
In the current study, Zhou, professor in the Verna and Marrs McLean Department of Biochemistry and Molecular Biology at Baylor, and her colleagues focused on necrosis, the type of cell death caused by injury or disease. Necrosis is mostly associated with stroke, cancer, neurodegenerative diseases and heart conditions. The team investigated in more detail the signal necrotic cells display that cues the 'eating' or phagocytic cells to engulf and eliminate the dying cell.
"In previous work, the Zhou lab showed that the signal in necrotic cells is phosphatidylserine, which is a type of lipid," said Yoshitaka Furuta, first author of the current study on which he worked while he was an undergraduate student intern in the Zhou lab. Furuta currently is a student in Baylor's Development, Disease Models and Therapeutics Graduate Program.
The team found that necrotic cells, but not living cells, expose phosphatidylserine on their outer surfaces and when phagocytes detect it, they ingest and destroy the dying cells."
Healthy cells keep phosphatidylserine in their inside, but when they are injured, for instance by lack of oxygen during a stroke when neurons are over excited due to constant entry of calcium ions, or during neurodegeneration, a process begins that ultimately flips phosphatidylserine to the outside of the cell. "We wanted to know what activates the flipping of phosphatidylserine," Furuta said.
Following the process of necrosis in the transparent worm C. elegans
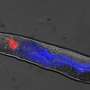
The researchers worked with the model organism C. elegans, a worm that is as long as a credit card is thick. C. elegans is transparent, which allows the researchers to visually identify necrotic neurons, which look swelled when compared to living cells, and follow changes in the dynamics of cellular components using a time-lapse recording approach they developed.
Previous work in C. elegans neurons had shown that certain mutations in ion channel proteins, which regulate the flow of ions like calcium2+ in and out of the cell, induced necrosis that was accompanied by an increase of calcium ion levels inside the cell. To investigate whether changes in calcium ion levels were linked to the triggering of the eat-me signal, the team introduced some of the ion channel mutant genes in neurons of their C. elegans model and monitored both calcium levels and phosphatidylserine over time.
"We discovered in our model of necrosis that a robust and transient increase in calcium ions inside the cell preceded phosphatidylserine exposure in necrotic neurons," said Zhou, a member of Baylor's Dan L Duncan Comprehensive Cancer Center. "Further experiments showed that necrotic neurons first had a small increase of calcium ions, which prompted the release of more calcium ions from an intracellular structure called the endoplasmic reticulum (ER), a larger source of calcium. Having larger than normal calcium levels inside the cell triggered phosphatidylserine exposure."
Supporting the role of calcium ions as a trigger of the 'eat-cell' signal, the researchers found that artificially increasing the level of calcium ions inside living cells also resulted in phosphatidylserine exposure and that suppressing calcium release from the ER prevented exposure of the signal.
The team also zoomed in into the process leading to the flipping of phosphatidylserine and found another piece of the puzzle. They discovered that a calcium increase inside the cells activates ANOH-1, an enzyme known to promote phosphatidylserine exposure.
"It was interesting to me that calcium can be good and bad for living cells," Furuta said. "Too much calcium is toxic to cells and induces necrosis, but at the same time calcium is necessary for dying cells to expose phosphatidylserine, the eat-me signal that promotes their clearance."
"Necrosis is a universal phenomenon that is present in many types of diseases and the clearance of necrotic cells is very important for keeping our bodies healthy," Zhou said.
Our findings provide new insight into the process of necrosis that can potentially lead to the development of therapeutic strategies, maybe along the lines of promoting clearance of necrotic cells by modulating phosphatidylserine exposure, which may help reduce the toxic effects of necrosis and improve cellular housekeeping."
More information: Yoshitaka Furuta et al, Calcium ions trigger the exposure of phosphatidylserine on the surface of necrotic cells, PLOS Genetics (2021). DOI: 10.1371/journal.pgen.1009066