'Bad fat' suppresses killer T cells from attacking cancer
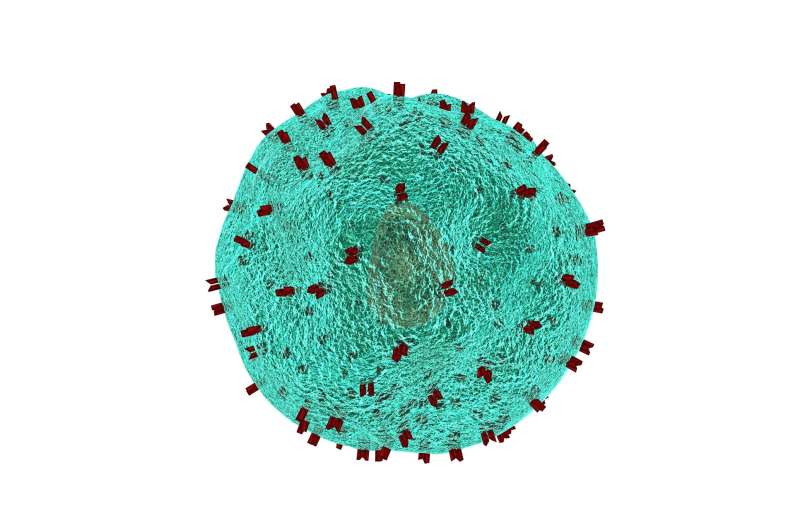
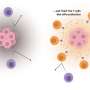
In order for cancer to grow and spread, it has to evade detection by our immune cells, particularly specialized "killer" T cells. Salk researchers led by Professor Susan Kaech have found that the environment inside tumors (the tumor microenvironment) contains an abundance of oxidized fat molecules, which, when ingested by the killer T cells, suppresses their ability to kill cancer cells. In a vicious cycle, those T cells, in need of energy, increase the level of a cellular fat transporter, CD36, that unfortunately saturates them with even more oxidized fat and further curtails their anti-tumor functions.
The discovery, published online in Immunity on June 7, 2021, suggests new pathways for safeguarding the immune system's ability to fight cancer by reducing the oxidative lipid damage in killer T cells. Identifying factors like these that cause immune suppression in the tumor microenvironment can lead to the development of novel immunotherapies for cancer.
"We know that tumors are a metabolically hostile environment for healthy cells, but elucidating which metabolic processes are altered and how this suppresses immune cell function is an important area of cancer research that is gaining a lot of attention," says Kaech, senior author and director of Salk's NOMIS Center for Immunobiology and Microbial Pathogenesis. "Our findings uncovered a novel mode of immunosuppression in tumors involving the import of oxidized fats (AKA lipids) in T cells via the cellular fat transporter CD36, which impairs their anti-tumor functions locally."
The burgeoning field of cancer immunometabolism studies how immune cell metabolism is reprogrammed within tumors and driven by alterations in nutrient availability. While scientists know that tumors accumulate fats—and that such accumulation is associated with immune dysfunction—the details of the relationship haven't been clear.
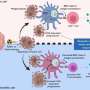
Working with Joseph Witztum's lab at UC San Diego and Antonio Pinto in the Salk Mass Spectrometry Core facility, the team established that tumors contain elevated amounts of several classes of lipid, and oxidized lipids in particular, which are generally found in oxidized low-density lipoproteins (LDLs), commonly considered "bad" fat. They then observed how killer T cells respond to the oxidized LDLs in tumors and found that killer T cells adapted to the tumor microenvironment by increasing CD36 on their surface and ingesting an abundance of oxidized lipids. Working with Brinda Emu's lab at Yale University, they found this process served as a catalyst to drive even greater amounts of lipid oxidation internally in the killer T cells and ultimately repressed their defenses.
Next, the team employed various methods to investigate how CD36 impaired killer T cell function. They created mouse models lacking CD36 on T cells and used antibodies to block CD36. They confirmed that CD36 promoted T cell dysfunction in tumors by increasing oxidized lipid import, which caused greater lipid oxidation and damage within the T cells and triggered the activation of a stress response protein, p38.
"We found that when the T cells get 'stressed out' by oxidized lipids, they shut down their anti-tumor functions," says Shihao Xu, a Salk postdoctoral fellow and the first author on the paper.
The team also found new therapeutic opportunities to reduce lipid oxidation and restore killer T cells' function in tumors through immunotherapy by blocking CD36 with an antibody therapy or by overexpressing glutathione peroxidase 4 (GPX4, a key molecule that removes oxidized lipids in cells).
Importantly, lipid oxidation doesn't just happen in T cells; it also happens in tumor cells, and too much of it can cause cell death. In fact, there is a lot of excitement in cancer research to increase lipid oxidation in tumor cells to a lethal level, but Kaech and her team urge some caution.
"Now that we've uncovered this vulnerability of T cells to lipid oxidation stress, we may need to find more selective approaches to inducing lipid oxidation in the tumor cells but not in the T cells," says Kaech, who holds the NOMIS Chair at Salk. "Otherwise, we may destroy the anti-tumor T cells in the process, and our work shows a few interesting possibilities for how to do this."
More information: Shihao Xu et al, Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors, Immunity (2021). DOI: 10.1016/j.immuni.2021.05.003