Researchers produce fully functional pancreatic beta cells from stem cells for the first time
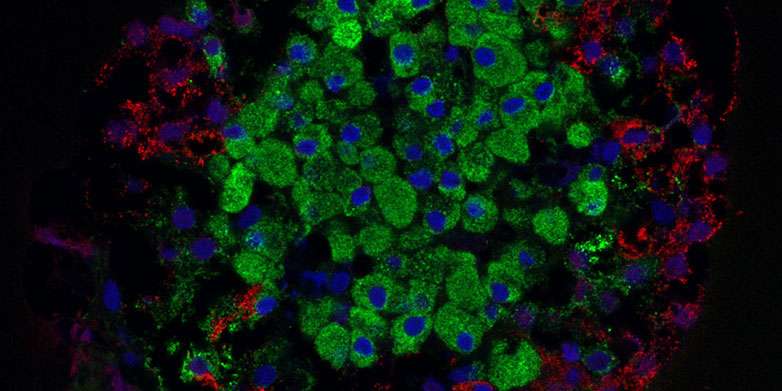
Insulin is a vital hormone produced by pancreatic beta cells. Type 1 diabetes is caused by the destruction of these cells, which results in patients having to replace the lost insulin with multiple daily injections.
Insulin secretion can be restored in diabetic patients by transplanting beta cells isolated from the pancreas of a brain dead organ donor. However, this treatment has not been widely introduced, since cells from at least two donors are needed to cure one diabetic.
For a long time, attempts have been made to produce functional beta cells from stem cells, which could make this treatment increasingly common. However, the beta cells produced from stem cells have so far been immature, with poorly regulated insulin secretion. This may be a partial explanation for why no breakthroughs have been achieved in the clinical trials based on immature cells ongoing in the United States.
A research group headed by Professor Timo Otonkoski at the University of Helsinki, has carried out pioneering efforts to optimize the functionality of pancreatic cells produced from stem cells.
In a recently published extensive article, the group has demonstrated, for the first time, that stem cells can form cells that closely mimic normal pancreatic islets, in terms of both structure and function.
The article was published in the Nature Biotechnology journal and it has been mainly funded by the Academy of Finland Center of Excellence MetaStem.
"In our study, insulin secretion was regulated as usual in cells, and the cells responded to changes in the glucose level even better than the pancreatic islets isolated from organ donors that were used as controls," says Väinö Lithovius, a member of the research group.
The most comprehensive survey of beta cell function
The researchers demonstrated the function of stem cell–derived beta cells in both cell cultures and mice studies. In the latter, the researchers demonstrated that stem cell–derived beta cells transplanted into mice started effectively managing the glucose metabolism of the mice.
"Blood glucose levels are higher in mice than in humans, roughly 8–10 millimolar. After the cell transplantation, the level decreased to that seen in humans, roughly 4–5 millimolar. It remained at this level, proving that the stem cell–derived transplant was capable of regulating blood glucose levels in mice," says researcher Jonna Saarimäki-Vire, who was responsible for the cell transplantation.
The survey of beta cell function now published is the most comprehensive in the field: in addition to insulin secretion, the researchers investigated the functionality of systems that regulate insulin secretion, including metabolism and ion channels, also connecting the findings to gene expression occurring during development.
"Our study will help further improve the production of stem cell islets, which will make it easier to utilize them in disease modeling and cell therapies," Timo Otonkoski says.
"I believe the international scholarly community values our efforts to validate stem cell islets as tools for diabetes research and cell therapies," says Professor Anders Tengholm from Uppsala University, who contributed to the study.
More information: Diego Balboa et al, Functional, metabolic and transcriptional maturation of stem cell derived beta cells, Nature Biotechnology (2021). DOI: 10.1101/2021.03.31.437748