Potential new treatments for Huntington's disease
Huntington's disease is caused by repeated toxic changes in the protein huntingtin. An interdisciplinary team from the Department of Molecular Neurology and the Department of Stem Cell Biology at Universitätsklinikum Erlangen and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) has now discovered a treatment that is currently undergoing clinical trials.
The researchers discovered that a small molecule accelerates the degradation of a specific messenger molecule necessary for protein synthesis in such a way that the huntingtin level in patients falls significantly.
A small molecule is an organic substance with a low molecular weight that is able to change biological processes such as protein synthesis. With this groundbreaking study, the interdisciplinary team from Erlangen was able to demonstrate for the first time how nerve cells of Huntington patients can be cured.
The results contribute to the long-term implementation of RNA-modifying approaches for fatal diseases such as Huntington's in clinical practice and the treatment of patients. The findings have recently been published in Nature Communications.
Huntington's disease is characterized by increasing involuntary and sometimes sudden movements. In addition to this restlessness, patients suffer from severe cognitive impairments and psychiatric symptoms. Most people fall ill at a young age and Huntington's disease is usually fatal 20 years after the onset. The reason for this is a specific change in the huntingtin gene, which is inherited with a probability of 50%. Genetically, there are repeated expansions of a small segment in the huntingtin gene, which are known as CAG repeats. These cause huntingtin to clump in the nerve cells, which irrevocably destroys them.
Therapeutic approaches that are currently being clinically tested attempt to lower the huntingtin level. Researchers from the Hereditary Movement Disorders Clinic at Universitätsklinikum Erlangen are also investigating this approach as part of a very early phase 2a clinical study.
Small molecules, in the case of the Erlangen researchers the active substance Branaplam, are administered as a tablet; they reduce the huntingtin level. This finding led to a first international study by the pharmaceutical company Novartis, which examined the effectiveness of Branaplam in Huntington's disease. However, the mechanism of action was unknown.
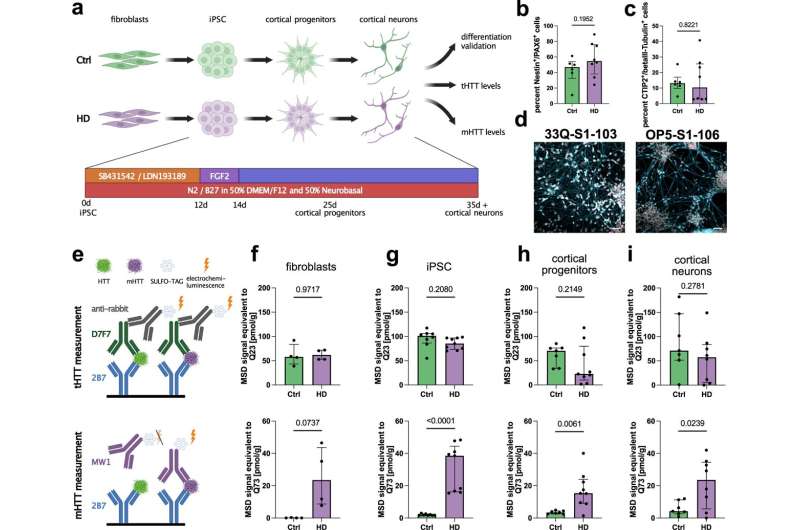
In order to uncover this in human nerve cells of Huntington patients, an interdisciplinary team from the fields of neurology, neuroscience and bioinformatics of molecular neurology and stem cell biology at Universitätsklinikum Erlangen joined forces. Brain nerve cells were produced in the laboratory using induced pluripotent stem cells, which are obtained from human skin cells and subsequently programmed to an early cell development stage. The team treated nerve cells from Huntington patients with Branaplam and discovered that the harmful huntingtin was reduced even after adding very small amounts of the active substance.
It was previously known that Branaplam binds to RNA messenger molecules. To investigate the effects of the active substance on the RNA messenger molecules, RNA molecules in many cell series were measured by means of RNA sequencing. Dr. Florian Krach from the Department of Stem Cell Biology succeeded in deciphering the mechanism of action in this enormous amount of data using biostatistical methods and artificial intelligence and found that Branaplam has a significant effect on the so-called splicing of RNA biomolecules. Unnecessary sections of the RNA messenger molecules are removed in the process.
This is also observed in the RNA messenger molecule of the huntingtin gene. The remaining section in the huntingtin RNA destabilizes and degrades the RNA messenger molecules, which means that the genetically modified huntingtin cannot develop in the first place.
More information: Florian Krach et al, An alternative splicing modulator decreases mutant HTT and improves the molecular fingerprint in Huntington's disease patient neurons, Nature Communications (2022). DOI: 10.1038/s41467-022-34419-x