This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Investigators capture a 'molecular snapshot' to illuminate the origins of pulmonary arterial hypertension
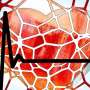
Pulmonary arterial hypertension (PAH) is a rare and incurable disease of the lung arteries that causes early death. In PAH, excess scar tissue and thickening of lung blood vessels occur as the result of increased cell "biomass." These changes obstruct blood flow and are detrimental to the heart, but until now the basic features of biomass in PAH were not known.
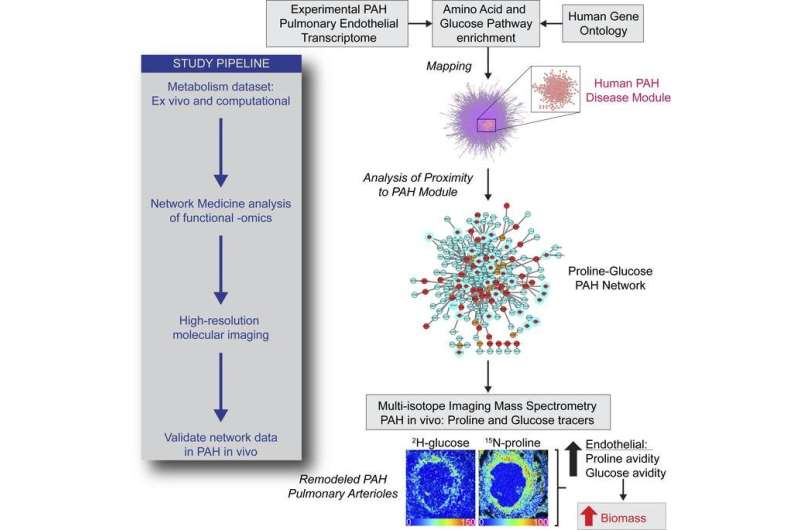
A team led by investigators at Brigham and Women's Hospital (BWH), a founding member of the Mass General Brigham healthcare system, in collaboration with Matthew Steinhauser, MD, a metabolism and cell imaging expert at the University of Pittsburg, and investigators at the University of Vienna, set out to better understand the origins of arterial biomass in PAH.
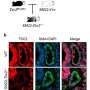
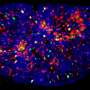
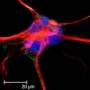
Using an animal model of PAH, the team applied network medicine and advanced molecular imaging tools to identify chemical building blocks that are taken up by arterial cells and ultimately contribute to blood vessel obstruction. Using multi-isotope imaging mass spectrometry (MIMS) under the guidance of Steinhauser and Christelle Guillermier, Ph.D., at BWH, the researchers could pinpoint the location and abundance of key contributors to biomass, including the amino acid proline and the sugar molecule glucose.
Using MIMS, the team visualized proline and glucose tracers injected into the bloodstream of an animal model of PAH. They saw that the molecules were used by arterial cells of the lung to build excess scar tissue (including the protein collagen), which contributed to blood vessel obstruction.
"Our study describes the world's first use of multi-isotope imaging mass spectrometry (MIMS) in the study of lung disease," said Bradley Wertheim, MD, of the Brigham's Division of Pulmonary and Critical Medicine. "MIMS is a powerful microscopy tool that produces a 'molecular snapshot' that can provide information down to the resolution of a single cell."
"These findings suggest that the uptake and metabolism of protein precursors may be fundamental to PAH biology. Closer investigation of proline and glucose in human PAH may uncover opportunities to inhibit biomass formation, prevent obstruction of lung arteries, and decrease the chance of heart failure for PAH patients," said co-senior author Bradley Maron, MD, of the Brigham's Division of Cardiovascular Medicine.
The research is published in the journal JCI Insight.
More information: Bradley M. Wertheim et al, Proline and glucose metabolic reprogramming supports vascular endothelial and medial biomass in pulmonary arterial hypertension, JCI Insight (2023). DOI: 10.1172/jci.insight.163932