This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers study infection and replication capacity of COVID in human respiratory organoids
A research team led by Dr. Zhou Jie and Professor Yuen Kwok-yung investigated the infection and replication capacity of the omicron BA.5 and other variants in human respiratory organoids established by the team. The findings were published in the latest issue of Proceedings of the National Academy of Sciences.
The emergence of SARS-CoV-2 new variants has caused multiple waves worldwide. Omicron was first detected in South Africa in November 2021, and it spread rapidly across the globe. The high transmissibility of SARS-CoV-2 omicron variants was largely ascribed to immune escape, which led to the significantly increased risk of reinfection or breakthrough infection in vaccinated populations.
However, important questions remained unresolved, that is, whether SARS-CoV-2 has evolved to gain increased replicative fitness during the past three years and whether omicron variants have become well-adapted in human respiratory cells. A biologically relevant model that can faithfully simulate the human respiratory epithelium is thus needed to investigate SARS-CoV-2 infection and replication in human respiratory cells.
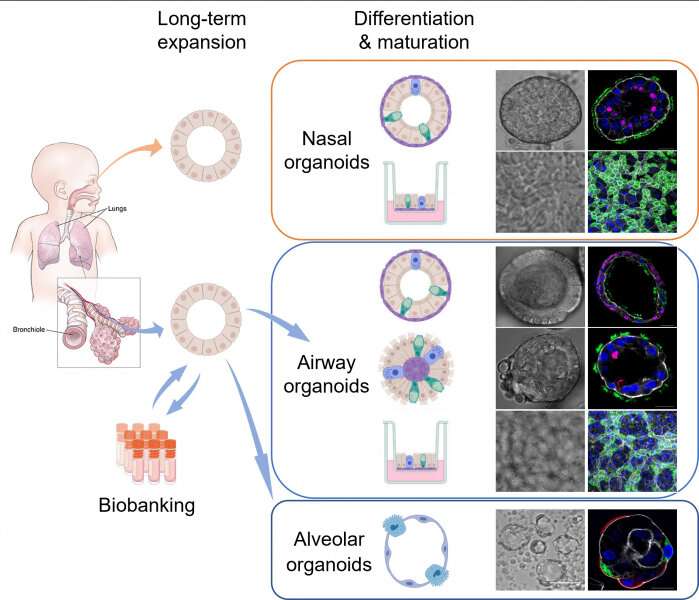
In collaboration with Professor Hans Clevers, Dr. Zhou Jie and Professor Yuen Kwok-yung's research team established the first organoid culture system of the human respiratory epithelium directly from primary lung tissues and nasal cells. These respiratory organoids were stably and consecutively expanded over six months to one year.
The research team induced maturation in long-term expandable organoids and generated nasal, airway and alveolar organoids that contain all major epithelial populations in the human respiratory tract. The brand-new organoid culture system enabled scientists to reconstruct and expand the entire human respiratory epithelium in culture dishes with excellent efficiency and stability, representing the optimal tools for studying respiratory biology and diseases, including COVID-19.
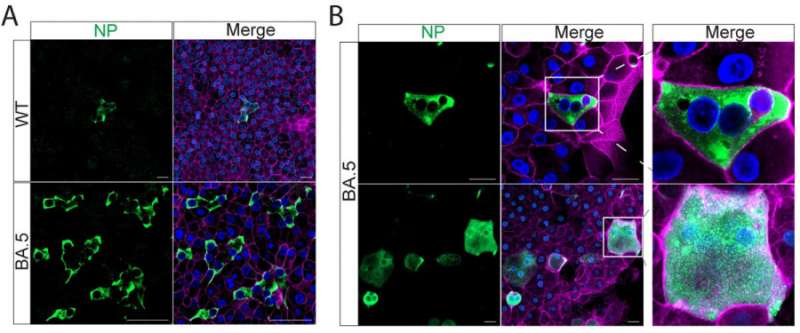
The research team then investigated the infection and replication of the ancestral strain of SARS-CoV-2 (WT), two omicron subvariants, B.1.1.529, and BA.5 in the airway and nasal organoids, and found that BA.5 exhibited a dramatically increased infectivity and replicative capacity than B.1.1.529 and the ancestral strain WT in human nasal and airway organoids.
BA.5 robustly replicated in human nasal and airway organoids to a comparable level to an influenza virus, pandemic H1N1 (H1N1pdm). Immunostaining and confocal microscopy revealed significantly more virus positive cells in BA.5 infected nasal and airway organoids than those in the organoids infected by the other two virus stains, B.1.1.529 and WT.
Interestingly, the team observed prominent syncytium formation in BA.5-infected nasal and airway organoids, albeit elusive in WT- and B.1.1.529- infected organoids. Overall, the higher entry efficiency and fusogenic activity of BA.5 enabled viral spread through syncytium formation in the human nasal and airway epithelial cells, leading to dramatically enhanced infectivity and transmissibility.
A lot of previous research has pointed out the high transmissibility of SARS-CoV-2 omicron variants was largely ascribed to immune escape. Dr. Zhou Jie and Professor Yuen Kwok-yung's team commented, "Based on the investigations in the human nasal and airway organoids established by our team, BA.5 has evolved to gain higher fitness in the human respiratory cells due to the enhanced entry efficiency and fusogenic activity."
The study has revealed a novel mechanism for the high transmissibility of BA.5 and provided scientific evidence for the implementation of public health policies. The respiratory organoids established by the team are robust and universal tools for studying respiratory biology and diseases.
More information: Cun Li et al, Human airway and nasal organoids reveal escalating replicative fitness of SARS-CoV-2 emerging variants, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2300376120