This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Central component of infection revealed in people living with HIV
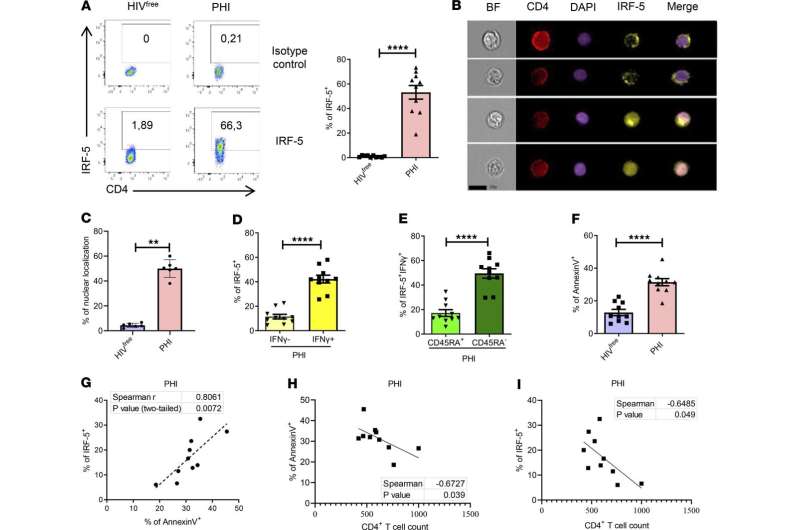
Professor Simona Stäger's team has made a breakthrough in the study of the human immunodeficiency virus (HIV). The researchers have identified the mechanism by which memory CD4+ T lymphocytes—cells that play a major role in our immune response—are predisposed to cell death in people living with HIV. The team's findings have just been published in JCI Insight.
In this new study, Professor Stäger and her research team built on work done on mice infected with the Leishmania donovani parasite (published in Cell Reports in 2018), which described how a chronic inflammatory environment predisposes certain cells to cell death. This premise led them to think that such a mechanism could be common to other chronic infectious diseases. They then turned their attention to HIV.
"This is an important discovery, since the cell death mechanism we demonstrated could be involved in other chronic infections like COVID-19 and visceral leishmaniasis in humans," says Stäger, Professor at INRS and expert in immunology of infectious diseases.
A molecular imprint leading to cell death
In most people living with HIV and undergoing antiretroviral treatment, residual inflammation persists despite some degree of viral control. The memory CD4+ T cells of people undergoing treatment are known to be prone to cell death. However, until today, the underlying mechanism remained largely unknown.
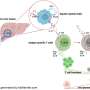
The researchers found that memory CD4+ T cells have a higher expression of the TLR7 receptor and the IRF5 transcription factor, which represents an imprint in these cells—predisposing them to cell death. The results also show that IRF-5 inhibitory peptides can block this predisposition.
The imprint Stäger's team observed is the consequence of a chronic inflammatory environment. The results of this study could therefore have implications for other chronic infectious diseases characterized by a significant inflammatory environment.
Memory cells: Keepers of immunity
Memory cells assist the immune system in protecting itself against a pathogen after an initial encounter with it. This is the basic principle of vaccines, which provoke a memory immune response—and therefore immunity—against an infectious agent. Consequently, the loss of these memory cells is a major risk for anyone suffering from a chronic infection.
In 80% of people living with HIV and receiving treatment, this discovery could help preserve memory cells. For the 20% of individuals in whom treatment remains ineffective, the breakthrough proposed by Professor Stäger's team could open the door to a more suitable therapy.
Liseth Carmona Perez, a Ph.D. student at INRS, spearheaded the project. Her research interests focus on infectious diseases and T lymphocytes, cells that play a major role in the human immune response. Liseth worked under the supervision of Professor Stäger and was accompanied by three other students: Linh Thuy Mai, Tanja Stögerer, and Sharada Swaminathan at the Armand-Frappier Santé Biotechnologie Research Centre.
"I feel very lucky to be able to work alongside such an incredible research group. I am hoping that our work will not only contribute to the advancement of medical knowledge, but also eventually lead to the development of new therapeutic approaches for managing chronic infectious diseases," says Liseth Carmona Perez, doctoral student in virology and immunology.
For this publication, Professor Simona Stäger and doctoral student Liseth Carmona Perez collaborated with the laboratory of Dr. Betsy Barnes of the Feinstein Institute for Medical Research in New York, along with leading HIV specialists: Dr. Jean-Pierre Routy of McGill University and his postdoctoral student Stéphane Isnard as well as Julien van Grevenynghe, professor at INRS and Xavier Dagenais-Lussier, his Ph.D. student at the time of the research.
More information: Liseth Carmona-Pérez et al, The TLR7/IRF-5 axis sensitizes memory CD4+ T cells to Fas-mediated apoptosis during HIV-1 infection, JCI Insight (2023). DOI: 10.1172/jci.insight.167329