How chronic infections can outsmart the immune system
Professor Simona Stäger and her team at INRS have discovered a mechanism that causes the body to sabotage its own defenses against infection by visceral leishmaniasis, a fatal tropical disease. This discovery could help in the fight against a number of chronic infections.
Like many chronic infections, visceral leishmaniasis thwarts the immune system's defenses so it can settle comfortably into its host. Responsible for tens of thousands of deaths around the world every year, visceral leishmaniasis holds the unfortunate distinction of being the second leading cause of death by parasitic infection, after malaria. But how does it slip through the host's defenses? Professor Simona Stäger's team at INRS has discovered a mechanism that Leishmania donovani takes advantage of to sustain the infection. It has shown that damage from chronic inflammation induces the death of white blood cells essential to eliminating the parasite. The findings, which were published in the prestigious journal Cell Reports, not only have the potential to lead to possible treatment, they also bring to light a phenomenon that may be shared by other chronic infections.
Weakened defenses
At the heart of the immune response, CD4 T cells are essential to controlling an infection. This type of white blood cell participates in the immune response by signaling the presence of a pathogen to be eliminated. To do this, CD4 T cells produce a molecule called interferon gamma (IFN-γ), which activates the cells that destroy pathogens.
In the case of visceral leishmaniasis, CD4 T cells expressing IFN-γ (Th1 cells) appear later than expected during infection and in smaller numbers, a phenomenon that had puzzled researchers. "Now we've found the reason why there are so few," said Professor Stäger. "Unfortunately, these protective cells are dying."
Discovery of a mechanism common to chronic infections
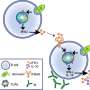
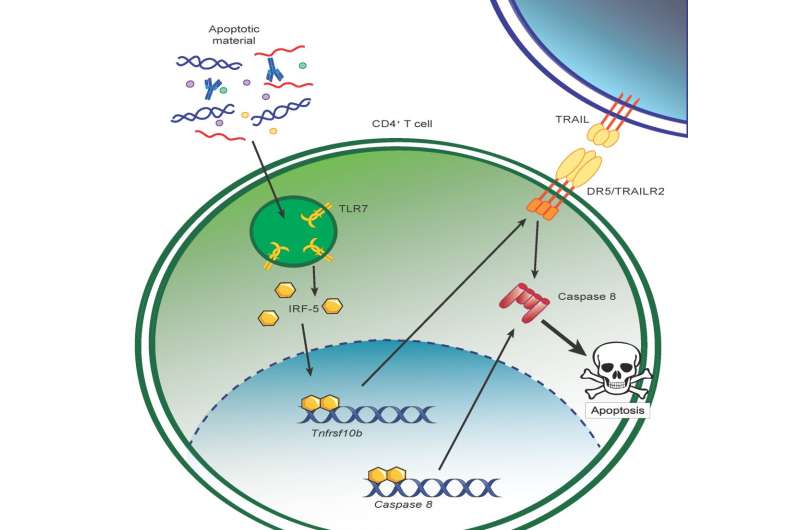
To understand how CD4 T cells are dying, researchers followed a lead identified in one of their recent studies. They had noticed the presence of a transcription factor unusual for these cells. A transcription factor is a protein that modulates the activity of one or more genes. The detected factor, IRF-5, is known for its action in innate immunity cells, but is role was totally unknown in CD4 T cells.
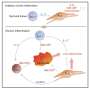
Professor Stäger's new findings show that IRF-5 leads Th1 cells to self-destruct. Tracing the thread of molecular events, the immunologist pinned down the trigger for this unexpected cell death: tissue destruction. Following a series of reactions, which are still not fully understood, the destroyed tissue activates previously unknown signals in Th1 cells, causing them to die.
The white blood cells that orchestrate the attack are thus eliminated—not by the parasite but by a biological process of the host—sustaining the chronic infection and protecting the parasite. In fact, this mechanism may also be at play in other chronic infections that lead to inflammation.
Given the importance of better understanding the workings of this process, INRS professor successfully secured $1 million in funding from the Canadian Institutes of Health Research. The cellular activity induced by IRF-5 in immune cells could reveal as-yet unknown therapeutic targets not only for visceral leishmaniasis, but also for other chronic inflammatory diseases.
Details of the signaling mechanism
The study presented by Professor Simona Stäger's team points to a mechanism involving the TLR7 receptor (Toll-like Receptor 7), usually activated in innate immune system cells by pathogen recognition. In the case of chronic inflammation, the cellular residues present after tissue destruction activate TLR7 in Th1 cells. Activation of TLR7 induces expression and activation of IRF-5, which in turn increases expression of DR5 (Death Receptor 5) and caspase 8, two signaling elements leading to cell death. Thus, chronic inflammation induces the death of protective CD4 T cells via the TLR7-IRF-5 cellular pathway.
More information: Aymeric Fabié et al, IRF-5 Promotes Cell Death in CD4 T Cells during Chronic Infection, Cell Reports (2018). DOI: 10.1016/j.celrep.2018.06.107