This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Immune and tumor cell 'tug-of-war' controls anti-cancer activity, study finds
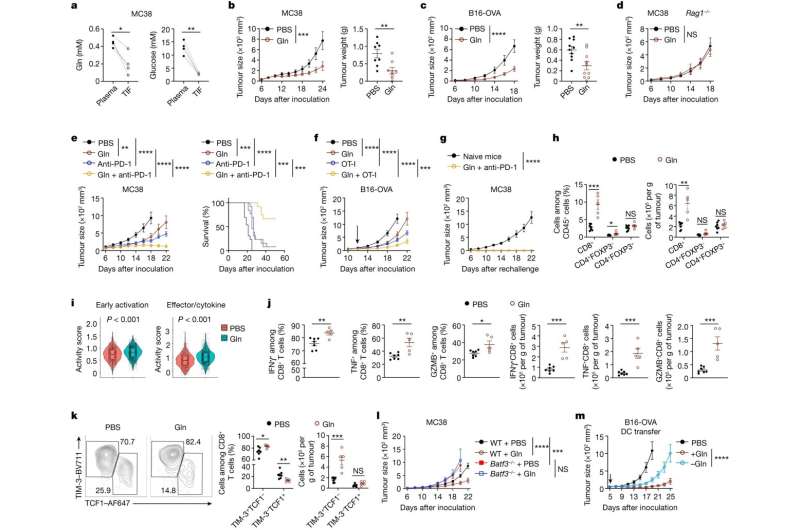
Scientists at St. Jude Children's Research Hospital found immune and tumor cells compete over glutamine, a major nutrient in their local environment, with significant implications for anti-cancer activity. If cancer cells monopolize glutamine, they can prevent immune cells from destroying cancer.
The findings show that supplying glutamine directly to tumors helps initiate the immune system's cancer-killing activity. The researchers also identified a molecular pathway that could serve as a potential drug target to achieve the same effect. The findings were published today in Nature.
"It's a nutrient tug-of-war between tumor cells and immune cells," said corresponding author Hongbo Chi, Ph.D., St. Jude Department of Immunology. "If tumor cells use all the available glutamine, then a specialized immune cell type known as the dendritic cell is starved of glutamine, leading to impaired anti-tumor immune function. But if we can supplement enough glutamine to the tumor microenvironment, that will inhibit tumor growth because dendritic cells will use it and activate the adaptive immune response."
Dendritic cells turn on cancer-killing immune cells called T cells.
The group showed that resupplying glutamine in the tumor microenvironment severely reduced tumor growth because dendritic cells were then better able to activate anti-cancer T cells. The tumor microenvironment is composed of chemicals and cells around cancer cells.
Infamously, cancer cells secrete many signals to turn the immune response "off" in this area, especially the T cells that threaten their destruction. The St. Jude team is the first to identify a nutrient as a major signal between cancer cells and dendritic cells in this local environment.
Improving anti-cancer therapies
"We are very excited to establish the link between glutamine, therapeutic effect and dendritic cells," said first author Chuansheng Guo, Ph.D., St. Jude Department of Immunology. "It's critical for the efficacy of immune checkpoint blockade and adoptive cell transfer therapy."
Immune checkpoint blockade therapy inhibits the "off" signals cancer cells send to immune cells that suppress the immune response in the tumor microenvironment. These therapies have been highly effective, but only in a small number of patients. The researchers found that supplying glutamine in combination with checkpoint therapy enhanced anti-cancer activity in mice.
"This paper provides a proof of concept that nutrients could act synergistically with checkpoint inhibitors for tumor treatment as a new strategy for combination therapy," Chi said.
The mechanism of helping or hindering cancer-killing
While much cancer research has focused on T cells because of their direct cancer-killing activity, this study is one of the first to examine how the tumor microenvironment affects the dendritic cells, which activate T cells. The researchers found that without glutamine, dendritic cells failed to activate the T cells that directly kill cancer cells.
"Even though T cells are the cornerstone for anti-cancer immunity, they cannot do the job by themselves," Chi explained. "We can think of dendritic cells as the driver and the T cell as the car. If you do not have a driver, the car will not move. Moreover, nutrients like glutamine serve as the license for the driver."
Similarly, when the researchers removed the proteins that respond to or take up glutamine in dendritic cells, the immune cells failed to activate cancer-killing cells. These proteins, called FLCN and SLC38A2, are important in sensing and acquiring nutrients but have not previously been connected to immune cells' reactions to tumors. They may serve as potent drug targets to improve cancer therapy.
"We're extremely excited about these findings," Chi said. "In addition to enhancing the therapeutic intervention, we have provided a conceptual advance by showing how nutrients mediate cell-cell communication, an understudied concept in the field of immunometabolism."
More information: Hongbo Chi, SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity, Nature (2023). DOI: 10.1038/s41586-023-06299-8. www.nature.com/articles/s41586-023-06299-8