This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research offers new approach to nongenetic T-cell-based immunotherapy
Immunotherapies for cancer aim to induce the immune system to combat cancer cells more effectively. In the journal Angewandte Chemie International Edition, a Chinese research team has now described a new, modular strategy for T-cell-based immunotherapy that manages to work without complex genetic modifications. Modulation of cell–cell communications through an ingenious regulatory circuit using various small, specially folded DNA molecules (aptamers) causes cancer cells to directly activate their mortal enemies, T cells.
For multicellular organisms like our bodies to function correctly, the cells must coordinate with one another. In this complex communications network, signals are sent and received, processed, and passed on. The regulation of specific membrane receptors that bind to signal molecules plays an important role in this process.
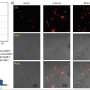
In a typical example, components of the immune system, known as antigen-presenting cells (APCs), sense the presence of cancer antigens. They transmit the signal to lymph nodes, in which specific T cells are activated by their receptors, move into the bloodstream, and kill the cancer cells. Unfortunately, cancer cells use a variety of loopholes to escape the immune system.
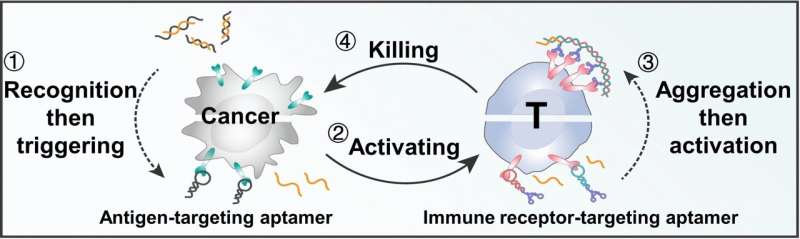
The team at Hunan University, Hangzhou Institute of Medicine, the Chinese Academy of Sciences, and Shanghai Jiao Tong University is working on ways to close these loopholes. Their goal is to establish new cellular interactions without having to produce genetically modified immune cells or receptors. The idea is to produce a short circuit in the communication pathways, by which the T cells are activated directly by the tumor cells, avoiding the detour through APCs.
Led by Weihong Tan and Liping Qiu, the team developed a regulatory circuit consisting of two modules: 1) recognition-then-triggering and 2) aggregation-then-activation. The circuit is based on different DNA aptamers—short DNA segments that fold into a preprogrammed 3D structure and recognize specific target molecules.
The DNA for module 1 is initially inactive and partially paired into a double strand. If cancer cells are present, the aptamer portion of the recognition single strand binds to protein tyrosinase kinase 7, a protein found in large numbers on the surface of many cancer cells. This splits the DNA double strand, releasing the triggering strand, which triggers module 2.
Module 2 requires two more types of aptamer. Both specifically bind to CD28 immunoreceptors on the surfaces of T cells. CD28 is a co-stimulator in the activation of T cells. This triggering strand binds to an additional loop on a type 1 aptamer. The loop opens and the newly released end binds to a type 2 aptamer, which then binds another type 1 aptamer, and so on (hybridization).
This results in a double strand and the bound CD28 receptors aggregate, triggering a signal cascade that massively amplifies the activation of T cells. In this way, short-circuited cell communication causes cancer cells to very effectively directly induce T cells to kill them.
More information: Zhimin Wang et al, An Aptamer‐Functionalized DNA Circuit to Establish an Artificial Interaction between T Cells and Cancer Cells, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202307656