This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Stem cell studies tune into hearing regeneration
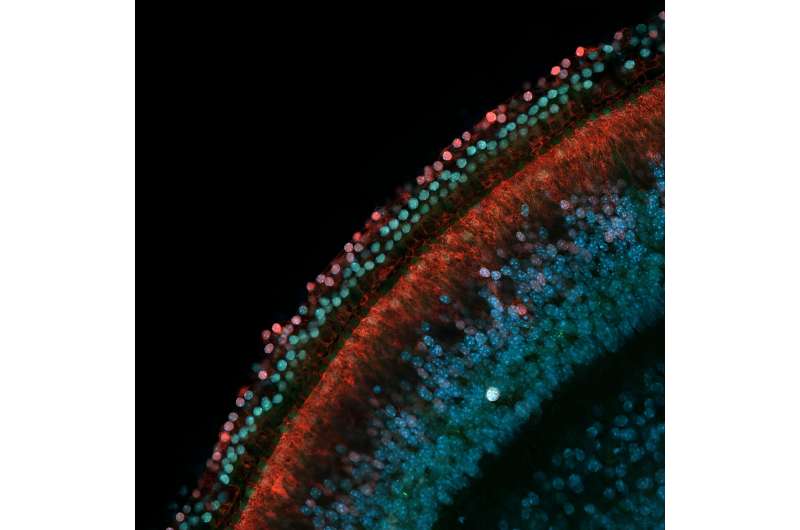
A deafened adult cannot recover the ability to hear, because the sensory hearing cells of the inner ear don't regenerate after damage. In two new studies, published in the Proceedings of the National Academy of the Sciences (PNAS), USC stem cell scientists explain why this is the case and how we might be able to change it.
"In the non-sensory supporting cells of the inner ear, key genes required for conversion to sensory cells are shut off through a process known as 'epigenetic silencing.' By studying how the genes are shut off, we begin to understand how we might turn them back on to regenerate hearing," said John Duc Nguyen, the first author of one of the papers.
The second paper explored when and how the ability to form sensory hearing cells is gained in the inner ear in the first place and describes two specific genes that could be useful for regenerating hearing in adults.
"We focused on the genes Sox4 and Sox11 because we found that they are necessary for forming sensory hearing cells during development," said the paper's first author Emily Xizi Wang.
One important way that genes are shut off or "silenced" involves chemical compounds called methyl groups that bind to DNA and make it inaccessible—the focus of Nguyen's paper. When the DNA that instructs a cell to become a sensory hearing cell is methylated, the cell cannot access these instructions.
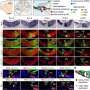
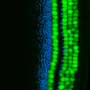
Through their experiments with non-sensory supporting cells extracted from the inner ears of mice, Nguyen and his colleagues confirmed that DNA methylation silences genes that promote conversion into sensory hearing cells, including the gene Atoh1 that is known to be a master regulator of inner ear development.
An enzyme called TET can remove methyl groups from the DNA, thus reversing the gene silencing and restoring the capacity of supporting cells to convert into sensory hair cells. Accordingly, when the scientists blocked the activity of TET, the supporting cells retained their DNA methylation and therefore could not convert into sensory hair cells in the petri dish.
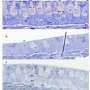
Intriguingly, in a separate experiment, the researchers tested the extent of gene silencing in supporting cells from a chronically deafened mouse. They found that gene silencing was partially reversed, meaning that the supporting cells had the capacity to respond to signals to transform into sensory hearing cells.
This finding has important implications: the loss of sensory hearing cells itself might partially reverse gene silencing in supporting cells in chronically deaf individuals. If so, the supporting cells of chronically deaf individuals might already be naturally primed to convert into sensory hearing cells.
In the second paper, Wang and her colleagues explored when and how the progenitor cells of the inner ear gain the ability to form sensory hearing cells.
The scientists pinpointed when progenitor cells acquire this ability: between days 12 and 13.5 of embryonic development in mice. During this window, the progenitor cells acquire the capacity to respond to signals from the master regulator gene Atoh1 that triggers the formation of sensory hearing cells later during development.
What primes the progenitor cells to respond to Atoh1 are two additional genes, Sox4 and Sox11, that change the state of these cells.
In embryonic mice lacking Sox4 and Sox11, progenitor cells in the inner ear failed to develop into sensory hearing cells. Specifically, a loss of Sox4 and Sox11 made the cells' DNA inaccessible—an effect similar to DNA methylation. With their DNA inaccessible, the progenitor cells couldn't respond to signals from Atoh1.
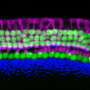
On the flip side, high levels of Sox4 and Sox11 activity stimulated mouse progenitor cells and supporting cells to form sensory hearing cells in a petri dish.
More promising still, in mice with damaged sensory cells in the inner ear, high levels of Sox4 and Sox11 activity increased the percentage of vestibular supporting cells that converted into sensory receptor cells—from 6 percent to 40%.
"We're excited to continue exploring the mechanisms by which cells in the inner ear gain the ability to differentiate as sensory cells during development and how these can be used to promote the recovery of sensory hearing cells in the mature inner ear," said the paper's corresponding author Ksenia Gnedeva, who is an assistant professor in the USC Tina and Rick Caruso Department of Otolaryngology—Head and Neck Surgery, and the Department of Stem Cell Biology and Regenerative Medicine.
More information: John D. Nguyen et al, DNA methylation in the mouse cochlea promotes maturation of supporting cells and contributes to the failure of hair cell regeneration, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2300839120
Wang, Xizi et al, SoxC transcription factors shape the epigenetic landscape to establish competence for sensory differentiation in the mammalian organ of Corti, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2301301120. doi.org/10.1073/pnas.2301301120